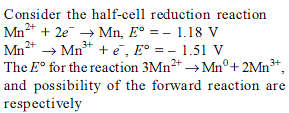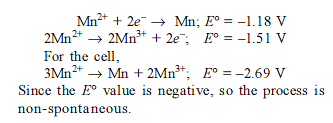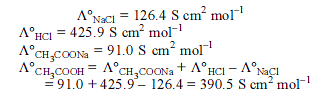JEE Exam > JEE Tests > Daily Test for JEE Preparation > Test: Electrochemistry(14 Oct) - JEE MCQ
Test: Electrochemistry(14 Oct) - JEE MCQ
Test Description
15 Questions MCQ Test Daily Test for JEE Preparation - Test: Electrochemistry(14 Oct)
Test: Electrochemistry(14 Oct) for JEE 2024 is part of Daily Test for JEE Preparation preparation. The Test: Electrochemistry(14 Oct) questions and answers have been
prepared according to the JEE exam syllabus.The Test: Electrochemistry(14 Oct) MCQs are made for JEE 2024 Exam. Find important
definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Test: Electrochemistry(14 Oct) below.
Solutions of Test: Electrochemistry(14 Oct) questions in English are available as part of our Daily Test for JEE Preparation for JEE & Test: Electrochemistry(14 Oct) solutions in
Hindi for Daily Test for JEE Preparation course. Download more important topics, notes, lectures and mock
test series for JEE Exam by signing up for free. Attempt Test: Electrochemistry(14 Oct) | 15 questions in 30 minutes | Mock test for JEE preparation | Free important questions MCQ to study Daily Test for JEE Preparation for JEE Exam | Download free PDF with solutions
Detailed Solution for Test: Electrochemistry(14 Oct) - Question 1
Detailed Solution for Test: Electrochemistry(14 Oct) - Question 2
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Detailed Solution for Test: Electrochemistry(14 Oct) - Question 3
Detailed Solution for Test: Electrochemistry(14 Oct) - Question 4
Detailed Solution for Test: Electrochemistry(14 Oct) - Question 5
Detailed Solution for Test: Electrochemistry(14 Oct) - Question 6
Detailed Solution for Test: Electrochemistry(14 Oct) - Question 7
Detailed Solution for Test: Electrochemistry(14 Oct) - Question 8
Detailed Solution for Test: Electrochemistry(14 Oct) - Question 9
Detailed Solution for Test: Electrochemistry(14 Oct) - Question 10
*Multiple options can be correct
Test: Electrochemistry(14 Oct) - Question 11
For a cell given below Ag | Ag+ || Cu2+ | Cu
— +
Ag+ + e- → Ag, Eº = x
Cu2+ +2e- → Cu, Eº = y
Eº cell is –
[AIEEE-2002]
Detailed Solution for Test: Electrochemistry(14 Oct) - Question 11
Test: Electrochemistry(14 Oct) - Question 12
Aluminium oxide may be electrolysed at 1000ºC to furnish aluminium metal (At. Mass=27 amu ; 1 Faraday = 96,500 Coulombs). The cathode reaction is
Al3+ + 3e-→ Alº
To prepare 5.12 kg of aluminium metal by this method would require -
[AIEEE-2005]
Detailed Solution for Test: Electrochemistry(14 Oct) - Question 12
Test: Electrochemistry(14 Oct) - Question 13
Given EºCr3+/Cr = – 0.72 V, EºFe2+/Fe= – 0.42 V. The potential for the cell Cr |Cr3+ (0.1 M)| |Fe2+ (0.01 M) | Fe is -
[AIEEE 2008]
Detailed Solution for Test: Electrochemistry(14 Oct) - Question 13
Detailed Solution for Test: Electrochemistry(14 Oct) - Question 14
Detailed Solution for Test: Electrochemistry(14 Oct) - Question 15
|
360 tests
|
Information about Test: Electrochemistry(14 Oct) Page
In this test you can find the Exam questions for Test: Electrochemistry(14 Oct) solved & explained in the simplest way possible.
Besides giving Questions and answers for Test: Electrochemistry(14 Oct), EduRev gives you an ample number of Online tests for practice