Test: Electrolysis & Faraday's Law(12 Oct) - JEE MCQ
10 Questions MCQ Test Daily Test for JEE Preparation - Test: Electrolysis & Faraday's Law(12 Oct)
Only One Option Correct Type
This section contains 16 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct
Q.
During the electrolysis of aqueous Zn(NO3)2 solution
Refining of impure metal is done by electrolysis using impure metal as anode.Select the correct statement about this refining.
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
In the electrosynthesis,potassium manganate (VII) is converted to manganese(IV) dioxide. By passage of 1F of electrolysis ,one mole of potassium manganate(VII) will form manganese dioxide.
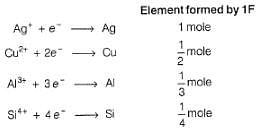
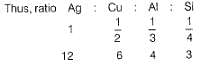
1 Faraday of electricity is passed through the solution containing 1 mole each of AgNO3, CuSO4, AlCl3 and SiCl4. Elements are discharged at the cathode.Number of moles of Ag, Cu,Al and Si formed will be in the ratio of
100 mL of a buffer of 1 M NH3(aq) and 1 M NH4+(aq) are placed in two volatic cells separately.A current of 1.5 A is passed through both cells for 20 min. If electrolysis of water only takes place
2H2O +O2 + 4e- → 4OH- (RHS
2H2O → 4H+. + O2 + 4e- (LHS)
then pH of the
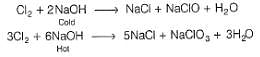
What product are formed during the electrolysis of a concentrated aqueous solution of sodium chloride using an electrolytic cell in which electrodes are separated by a porous pot?
I. Cl2(g)
II. NaOH(aq)
III. H2(g)
IV. NaClO(aq)
V. NaClO3(aq)
Select the correct choice.
Select the correct statement(s) about electrolysis of aqueous CuSO4 solution.
Which pair of electrolysis could be distinguished by the products of electrolysis using inert electrodes?
An electrochemical cell was based on the following reaction:
Mn(OH)2(s) + H2O2(aq) → MnO2(s) + 2H2O (e)
During the opeartion of this for 1 min, 0.135 g of MnO2 was produced. What is the average electric current (in ampere ) produced by the cell?
A fully charged battery contains 500 mL of 5.00 M H2SO4. What is the concentration of H2SO4 in the battery after 6.0 A of current is drawn from the battery for 13.40 h?
|
360 tests
|


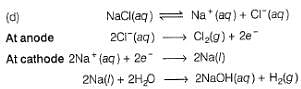
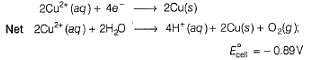 since, E0cell < 0, in electrolysis.
since, E0cell < 0, in electrolysis.














