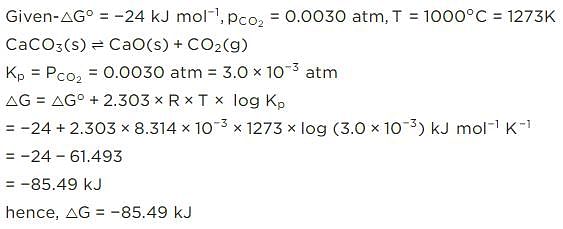Test: Free Energy Change & Spontaneity - NEET MCQ
15 Questions MCQ Test Chemistry Class 11 - Test: Free Energy Change & Spontaneity
Direction (Q. Nos. 1- 8) This section contains 8 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. For the given reaction, 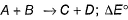 = - 1.3818 kcal at 300 K. Thus equilibrium constant is
= - 1.3818 kcal at 300 K. Thus equilibrium constant is
ΔHvap = 30 kJ mol-1 and ΔSvap = 75 J mol-1 K-1. Thus, temperature of vapour at one atmosphere is
[IIT JEE 2004]
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which reaction, with the following values of ΔH and ΔS at 400 K is spontaneous and endothermic?
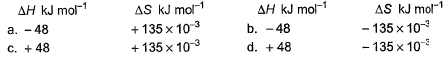
Standard entropies of X2, Y2 and XY3 are given below the reaction
Q. At what temperature, reaction would be in equilibrium?
The value of log10 K for a reaction, A B is (Given, ΔH°298 = - 54.07 kJ mol-1;
ΔS°298 = + 10 JK-1 mol-1; R = 8.314 JK-1 mol-1 2.303 x 8.314 x 298 = 5705)
[IITJEE2007]
For the following decom position reaction,
ΔG° = 1700 J mol-1 at 290 K , (log 5 = 0.6990]
Thus, pressure set up is
If for the cell, Zn(s) + Cu2+(ag) Cu(s) + Zn2+ (ag)entropy change ΔS° is 96.5 JK-1 mol-1, then temperature coefficient of the emf of a cell is
For the reaction, at 1000° C
ΔG° = - 24 kJ mol-1, 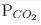 = 0.0030 atm.
= 0.0030 atm.
Q. Hence, ΔG at this temperature is
Direction (Q. No. 9) This sectionis based on statement I and Statement II. Select the correct answer from the code given below.
Statement I :Every endothermic reaction is spontaneous if TΔS > ΔH.
Statement II : Sign of ΔG is the true criterion for deciding spontaneity of a reaction.
Direction (Q. Nos. 10 and 11) This section contains 2 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THANT ONE is correct.
Q. Which of the following statements is/are true?
When HCI(g)and NH3(g)come in contact, they react producing a white cloud of solid NH4CI
For this,
Direction (Q. Nos. 11-14) This section contains 2 paragraph, wach describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given options (a),(b),(c),(d).
Passage l
Sulphur undergoes a phase transition between 80 and 110°C
S(rhombic) S (monoclinic); ΔH° = 3.213 kJ mol-1; ΔS° = 8.71 JK-1 mol-1
Q. Select the correct alternate(s).
Passage l
Sulphur undergoes a phase transition between 80 and 110°C
S(rhombic) S (monoclinic); ΔH° = 3.213 kJ mol-1; ΔS° = 8.71 JK-1 mol-1
Q. Temperature at which ΔG° = 0, is
Q. Select the correct alternate.
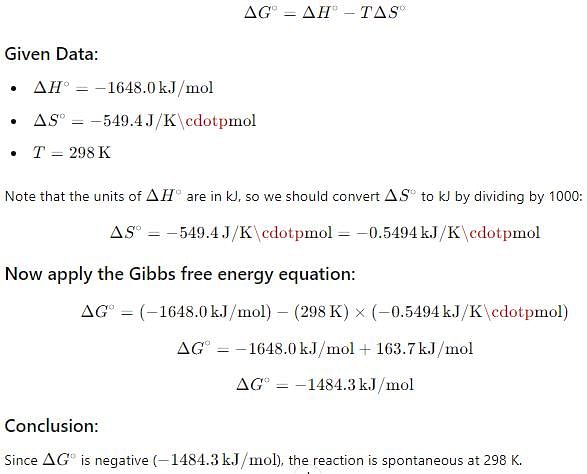
For oxidation of iron at 298 K, 4 Fe (s) + 3 O2(g) → 2 Fe2O3(s)
ΔS° = - 549.4 JK-1 mol-1 and ΔH ° = - 1648 . 0 kJ mol-1
Calculate the Gibbs free energy for the reaction of conversion of ATP into ADP at 293 Kelvin the change in enthalpy is 19.07 Kcal and the change in entropy is 90 cal per Kelvin.
|
127 videos|244 docs|87 tests
|