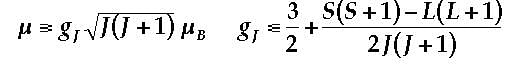Test: Lanthanide- 2 - Chemistry MCQ
30 Questions MCQ Test - Test: Lanthanide- 2
The incorrect statement among the following that describes the property of lanthanides is:
Consider the following statements:
(I) The size of the lanthanide M3+ ions decreases as the atomic number of M increases.
(II) Electronic spectra of lanthanides show very broad band.
(III) As with transition metals, coordination number six is very common in lanthanide complexes.
Which of the statements given above is/are correct:
(II) Electronic spectra of lanthanides show very broad band.
(III) As with transition metals, coordination number six is very common in lanthanide complexes.
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which one of the following is the correct sequence of Ce3+, La3+, Pm3+ and Yb3+ in increasing order of their ionic radii:
Magnet ic moment (μ) in BM of Pr3+ with outer electronic configuration is:
Ce3+ and Yb3+ are colourless but show strong absorption in UV region. This is due to:
The calculated ground state magnetic moment of Sm3+ at room temperature is:
Lanthanides are used for wavelength calibration of instruments because:
(I) f-orbitals are deep inside the atom.
(II) The absorption bands are sharp.
(III) Charge transfer spectra is possible.
The correct statements is/are
Match coordination number and shape with most probable example, of lanthanide ion choose the correct answer using the codes given below:
Which of the following compounds show a charge-transfer band:
The actual magnetic moment shows a large deviation from the spin-only formula in the case of:
The 3d element show variable oxidation states. What is the maximum oxidation state shown by the element Mn?
The metallic radii are abnormally high for which of the following pairs:
Among the following statements, ident ify the correct ones for complexes of lanthanide (III) ion:
Statement I: The size of Zr and Hf are similar
Statement II: Size of Hf is affected by lanthanide contraction.
The coordination number and geometry of cerium in [Ce(NO3)6]2– are respectively:
The pair of lanthanides with the highest third-io nization energy is:
The lanthanide(III) ion having the highest partition coefficient between tri-n-but ylphosphate and conc. HNO3 is:
Consider the ions Eu(III), Gd(III), Sm(III) and Lu(III). The observed and calculated magnetic moment values are closest for the pair:
Consider the following statements with respect to uranium:
(I) UO2+ disproportionate more easily than
(II) U3O8 is its most stable oxide of U
(III) Coordination number of U in [UO2(NO3)2(H2O)2].4H2O is six
(IV) is linear
The correct set of statements is
Consider the following statements for (NH4)2[Ce(NO3)6] (Z)
(I) Coordination number of Ce is 12
(II) Z is paramagnetic
(III) Z is an oxidizing agent
(IV) Reaction of Ph3PO with Z gives a complex having coordination number 10 for Ce.
The correct statements are
For uranocene, the correct statement(s) is/are:
(I) Oxidation state of uranium is ‘+4’
(II) It has cyclooctatetraenide ligands
(III) It is a bent sandwich compound
(IV) It has ‘–2’ charge
Correct answer is
Which of the following statements are true for the lanthanides:
(I) The observed magnetic moment of Eu3+ at room temperature is higher than that calculated form spin-orbit coupling.
(II) Lanthanide oxides are predominantly acidic in nature.
(III) The stability of Sm(II) is due to its half-filled sub-shell
(IV) Lanthanide(III) ions can be separated by ion exchange chromatography Correct answer is
The lanthanide complex (acec = acet ylacetonate; phen = 1, 10-phenanthroline that do not have square antiprismatic structure is:
The difference in the measured and calculated magnetic moment (based on spin-orbit coupling) is observed for:
The separation of trivalent lanthanide ions, Lu3+, Yb3+, Dy3+, Eu3+ can be effectively done by a cation exchange resin using ammonium o-hydroxy isobutyrate as the eluent. The order in which the ions will be separated is:
The calculated and observed magnetic moments differ considerably for an aqua complex of a lanthanide(III) ion as a result of low lying states of high J. The ion, among the following, is: