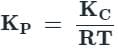Test: Law of Chemical Equilibrium and Equilibrium Constant (3 July) - JEE MCQ
10 Questions MCQ Test Daily Test for JEE Preparation - Test: Law of Chemical Equilibrium and Equilibrium Constant (3 July)
On increasing the pressure, in which direction will the gas phase reaction proceed to re-establish equilibrium, is predicted by applying the Le Chatelier’s principle. Consider the reaction.
N2 (g) + 3H2 (g) ⇌ 2NH3 (g)
Which of the following is correct, if the total pressure at which the equilibrium is established, is increased without changing the temperature?
N2 (g) + 3H2 (g) ⇌ 2NH3 (g)
Which of the following is correct, if the total pressure at which the equilibrium is established, is increased without changing the temperature?
If for 
 The equilibrium constants are k1 and k2 respectively, the reaction
The equilibrium constants are k1 and k2 respectively, the reaction  would have equilibrium constant
would have equilibrium constant

 The equilibrium constants are k1 and k2 respectively, the reaction
The equilibrium constants are k1 and k2 respectively, the reaction  would have equilibrium constant
would have equilibrium constant| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Naphthalene (C10H8) balls were kept in a closed container at room temperature (27°C). The vapour pressure above the balls was found to be 0.10 mm Hg. Find the value of Kc(sublimation)
For a reaction 2A ⇋ B + C, Kc is 2 × 10-3. At a given time, the reaction mixture has [A] = [B] = [C] = 3 × 10-3 M. Which of the following options is correct?
If the equilibrium constant for the reaction 2SO2 + O2 ⇋ 2SO3 is 64 at 500 K, then the equilibrium constant for the reaction SO3 ⇋ SO2 + (1/2)O2 at the same temperature is
Which of the following statements is correct?
If Kf and Kb represents the equilibrium constants of the forward and backward chemical reactions respectively of a particular reversible reaction then:
N2(g) + 3H2(g) ⇌ 2NH3(g)
At equilibrium, if the pressure is increased at a constant temperature, there will be an increase in the number of molecules of
For the reaction
4NH3(g)+7O2(g) ⇌ 6H2O(g)+4NO2(g), the value of Kp is-
For the given equilibrium reaction.
2 A (g) ⇌ 2 B (g) + C (g)
the equilibrium constant (Kc) at 1000 K is 4 × 10-4 Calculate Kp for the reaction at 800 K temperature
|
360 tests
|


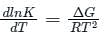
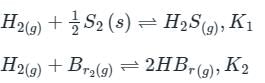
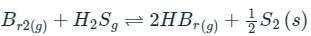
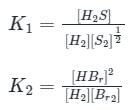
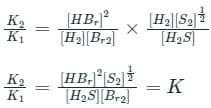
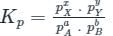
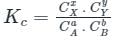
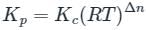
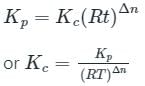
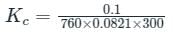
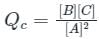
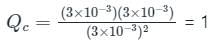
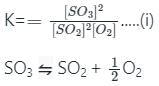
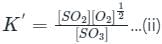
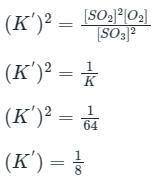
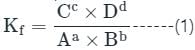
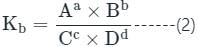
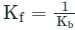
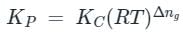
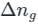 is the number of products - number of moles of reactants
is the number of products - number of moles of reactants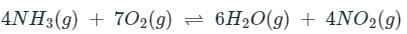
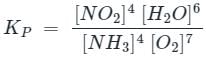
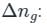

 value is
value is