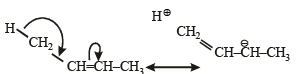Test: MCQs (One or More Correct Option): Organic Chemistry - Some Basic Principles & Technique | JEE Advanced - JEE MCQ
18 Questions MCQ Test 35 Years Chapter wise Previous Year Solved Papers for JEE - Test: MCQs (One or More Correct Option): Organic Chemistry - Some Basic Principles & Technique | JEE Advanced
Resonance structures of a molecule should have :
Phenol is less acidic than :
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Dipole moment is shown by :
Only two isomeric monochloro derivatives are possible for:
Which of the following have asymmetric carbon atom?
What is the decreasing order of strength of the bases 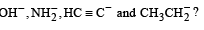
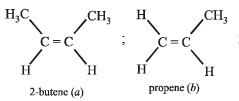
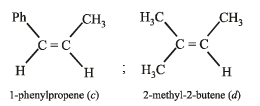
Which of the following compounds will show geometrical isomerism?
Among the following compounds, the strongest acid is
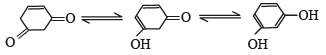
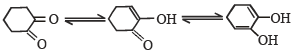
Tautomerism is exhibited by
An aromatic molecule will
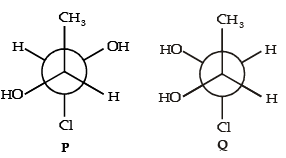
The correct statements(s) concerning the structures E,F and G is (are) –
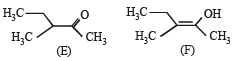
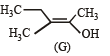
The correct statement(s) about the compound given below is (are)
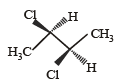
The correct statement(s) about the compound
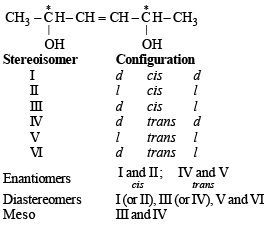
H3C(HO)HC–CH = CH – CH(OH)CH3 (X) is(are)
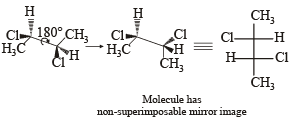
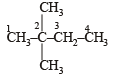
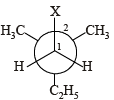
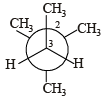
In the Newman projection for 2,2-dimethylbutane
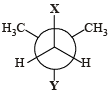
X and Y can respectively be
Amongst the given options, the compound(s) in which all the atoms are in one plane in all the possible conformations (if any), is (are)
Which of the following molecules, in pure form, is (are) unstable at room temperature ?
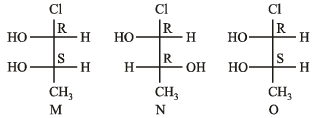
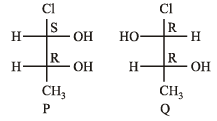
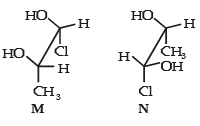
Which of the given statement(s) about N, O, P and Q with respect to M is (are) correct ?
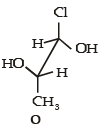
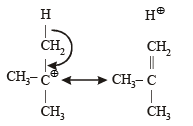
The hyperconjugative stabilities of tert-butyl cation and 2-butene, respectively, are due to
|
347 docs|185 tests
|
|
347 docs|185 tests
|


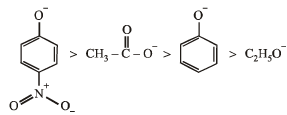
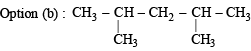 will give three isomers with Cl group at either of the CH3 groups, second C-atom and 3rd C-atom.
will give three isomers with Cl group at either of the CH3 groups, second C-atom and 3rd C-atom.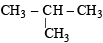 will again give two isomers with Cl at either one of the CH3 groups or on the central C-atom
will again give two isomers with Cl at either one of the CH3 groups or on the central C-atom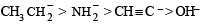
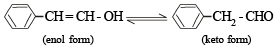
 Tautomerism is not possible
Tautomerism is not possible