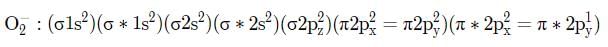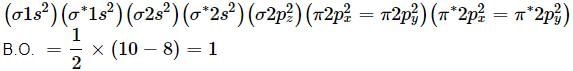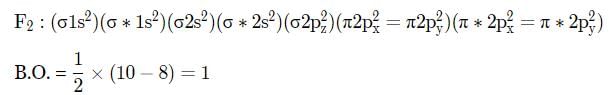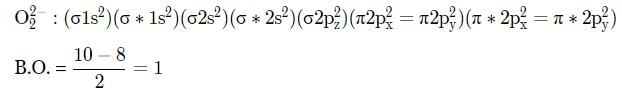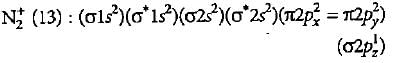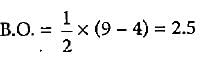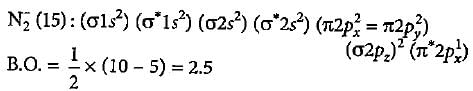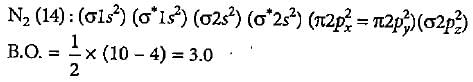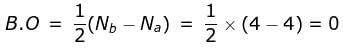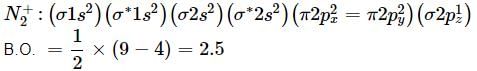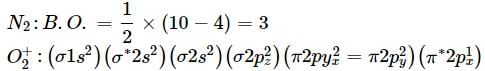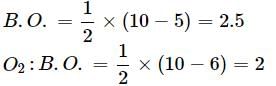Test: Molecular Orbital Theory (NCERT) - NEET MCQ
15 Questions MCQ Test NCERTs at Fingertips: Textbooks, Tests & Solutions - Test: Molecular Orbital Theory (NCERT)
Which of the following statements is not true regarding molecular orbital theory?
2s and 2p-atomic orbitals combine to give how many molecular orbitals?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The conditions for the combination of atomic orbitals to form molecular orbitals are stated below. Mark the incorrect condition mentioned here.
The electronic configuration of carbon is Is2 2s2 2p2. There are 12 electrons in C2. The correct electronic configuration of C2 molecule is
The increasing order of energies of various molecular orbitals of N2 is given below:
σls <σ*ls <σ2s <σ*2s <π2px = π2py <σ2pz < π*2px = π*2py<σ*2pz
The above sequence is not true for the molecule
Which of the following species has unpaired electrons?
What is the order of stability of N2 and its ions?
What will be the bond order of the species with electronic configuration 1s2 2s2 2p5?
Which of the following bond orders is indication of existence of a molecule?
Which of the following pairs will have same bond order?
According to molecular orbital theory, which of the following will not exist?
Which of the following facts regarding bond order is not valid?
Which of the following formulae does not show the correct relationship?
Fill in the blanks with the appropriate choice.
Bond order of N+2 is ___P___ while that of N2 is ___Q___ . Bond order of O+2 is ___R___ while that of O2 is ___S___ . N−N bond distance ___T___ , when N2 changes to N+2 and when O2 changes to O+2 , the O-O bond distance ___U___ .
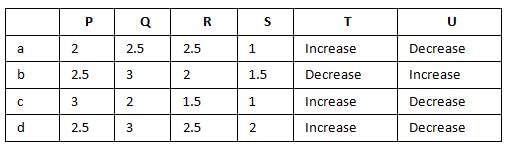
|
257 docs|234 tests
|
|
257 docs|234 tests
|