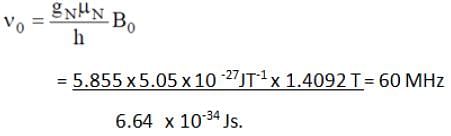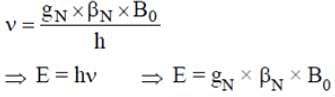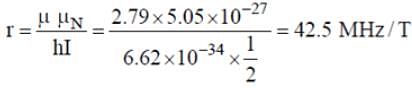Test: NMR Spectroscopy - Chemistry MCQ
10 Questions MCQ Test Organic Chemistry - Test: NMR Spectroscopy
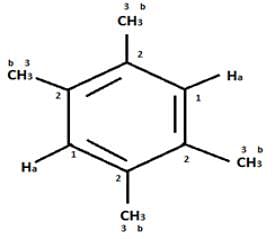
An organic compound having the molecular formulae C10H14 exhibited two singlets in the 1H NMR spectrum and three signals in the 13C NMR. What is the compound?
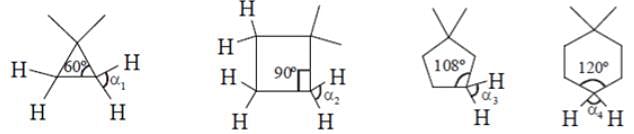
What will be the strength of coupling between geminal protons in the following molecules?

| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
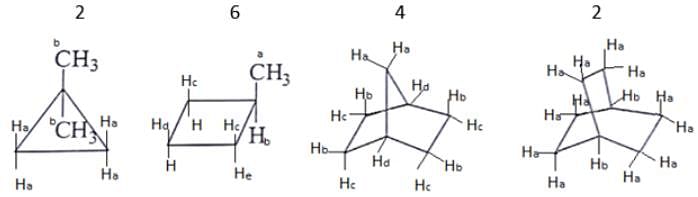
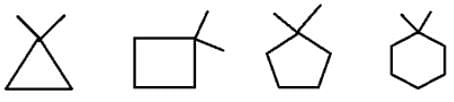
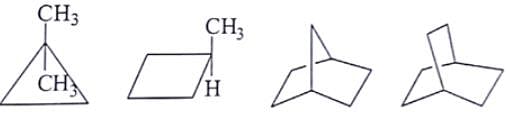
What are the number of signals in 1H NMR in the given molecules?

What will be the NMR frequency in MHz of bare 1H in a magnetic field of intensity 1.4092 tesla (given gN = 5.585 and μN = 5.05 x 10-27 JT-1)?
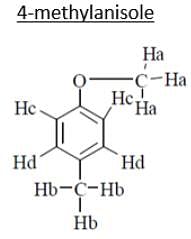
An organic compound (MF; C8H10O) exhibited the following 1H NMR special data: 62.5 (3H, s), 3.8 (314, s), 6.8 (2H, d, J 8 Hz), 7.2 (2H, d, J 8 Hz) ppm. What will be the compound among the choices?
In NMR spectroscopy, what is the product of Nuclear ‘g’ factor (gN), the nuclear magneton and the magnetic field strength (B0)?
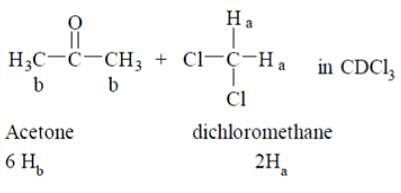
The 1H NMR spectrum of a dilute solution of a mixture of acetone and dichloromethane in CDCl3 exhibits two singlets of 1:1 intensity. What will be the molar ratio of acetone to dichloromethane in the solution?
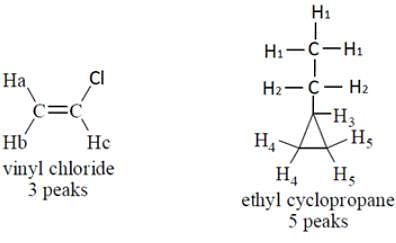
How many peaks are expected in low-resolution NMR spectrum of vinyl chloride and ethyl cyclopropane?
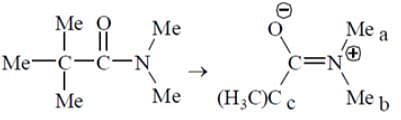
At room temperature, what is the number of singlet resonances observed in the 1H NMR spectrum of Me3CC(O)NMe2 (N, N-Dimethylpivalamide)?
|
35 videos|92 docs|46 tests
|