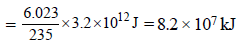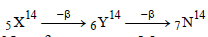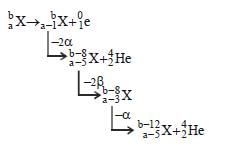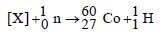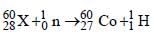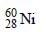Test: Nuclear Chemistry - From Past 31 Years Questions - NEET MCQ
20 Questions MCQ Test Chemistry 31 Years NEET Chapterwise Solved Papers - Test: Nuclear Chemistry - From Past 31 Years Questions
The half life of a radioactive nuclide is 100 hours. The fraction of original activity that will remain after 150 hours would be: [2021]
The half-life of a radioactive sample undergoing α-decay is 1.4 × 1017 sec. If the number of nuclei in the sample is 2.0 × 1021, the activity of the sample is nearly equal to: [2021]
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The half life of a substance in a certain enzymecatalysedreaction is 138s. The time required forthe concentration of the substance to fall from1.28 mg L–1 to 0.04 mg L–1, is : [2011]
A nuclide of an alkaline earth metal undergoesradioactive decay by emission of the α-particles in succession. The group of theperiodic table to which the resulting daughterelement would belong is [2005]
The radioactive isotope, tritium, 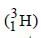 has a halflifeof 12.3 years. If the initial amount of tritium is32 mg, how many milligrams of it would remainafter 49.2 years? [2003]
has a halflifeof 12.3 years. If the initial amount of tritium is32 mg, how many milligrams of it would remainafter 49.2 years? [2003]
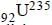 nucleus absorbs a neutron and disintegrates into
nucleus absorbs a neutron and disintegrates into 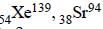 and x. So what will be the product x? [2002]
and x. So what will be the product x? [2002]
A human body required 0.01M activity ofradioactive substance after 24 hours. Half life ofradioactive substance is 6 hours. Then injectionof maximum activity of radioactive substance thatcan be injected will be [2001]
When a radioactive element emits successivelyone α-particle and two β-particles, the massnumber of the daughter element [1999]
Carbon - 14 dating method is based on the factthat: [1997]
One microgram of radioactive sodium 2411Na witha half-life of 15 hours was injected into a livingsystem for a bio-assay. How long will it take forthe radioactive subtance to fall up to 25% of theinitial value? [1996]
Half-life for radioactive 14C is 5760 years. In howmany years, 200 mg of 14C will be reduced to 25mg? [1995]
India has the world’s largest deposits of thoriumin the form of [1994]
In a radioactive decay, an emitted electron comesfrom [1994]
If an isotope of hydrogen has two neutrons in itsatom, its atomic number and atomic mass numberwill respectively be [1992]
Emission of an alpha particle leads to a
[1989]
The age of most ancient geological formations is estimated by
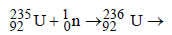
fission products + neutrons + 3.20 × 10–11 J The energy released when 1 g of finally undergoes fission is
Number of neutrons in a parent nucleus X, which gives 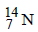 nucleus, after two successive β emissions, would be
nucleus, after two successive β emissions, would be
If species 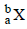 emits firstly a positron, then two α and two β and in last one α and finally converted to species
emits firstly a positron, then two α and two β and in last one α and finally converted to species 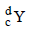 , so correct relation is
, so correct relation is
The radioactive isotope 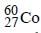 which is used in the treatment of cancer can be made by (n, p) reaction. For this reaction the target nucleus is
which is used in the treatment of cancer can be made by (n, p) reaction. For this reaction the target nucleus is
|
29 docs|49 tests
|
|
29 docs|49 tests
|


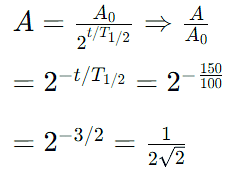
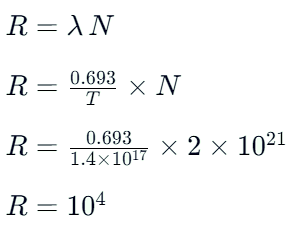
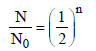
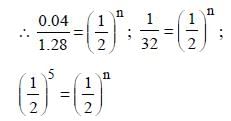
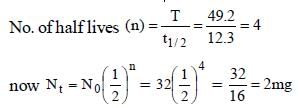

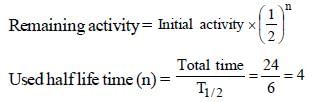

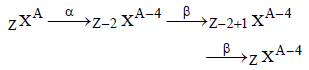
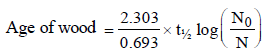
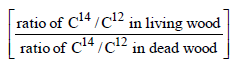
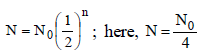
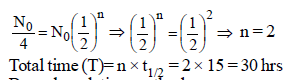
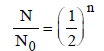
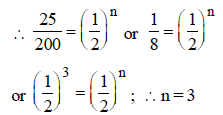
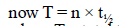
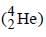 leads to
leads to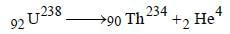
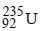 on fission gives energy
on fission gives energy