Class 10 Exam > Class 10 Tests > Extra Documents, Videos & Tests for Class 10 > Test: Periodic Classification Of Elements (Medium) - Class 10 MCQ
Test: Periodic Classification Of Elements (Medium) - Class 10 MCQ
Test Description
7 Questions MCQ Test Extra Documents, Videos & Tests for Class 10 - Test: Periodic Classification Of Elements (Medium)
Test: Periodic Classification Of Elements (Medium) for Class 10 2024 is part of Extra Documents, Videos & Tests for Class 10 preparation. The Test: Periodic Classification Of Elements (Medium) questions and answers have been
prepared according to the Class 10 exam syllabus.The Test: Periodic Classification Of Elements (Medium) MCQs are made for Class 10 2024 Exam. Find important
definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Test: Periodic Classification Of Elements (Medium) below.
Solutions of Test: Periodic Classification Of Elements (Medium) questions in English are available as part of our Extra Documents, Videos & Tests for Class 10 for Class 10 & Test: Periodic Classification Of Elements (Medium) solutions in
Hindi for Extra Documents, Videos & Tests for Class 10 course. Download more important topics, notes, lectures and mock
test series for Class 10 Exam by signing up for free. Attempt Test: Periodic Classification Of Elements (Medium) | 7 questions in 7 minutes | Mock test for Class 10 preparation | Free important questions MCQ to study Extra Documents, Videos & Tests for Class 10 for Class 10 Exam | Download free PDF with solutions
Test: Periodic Classification Of Elements (Medium) - Question 1
An atom has the symbol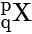 .Which value determines the position of the element in the periodic table?
.Which value determines the position of the element in the periodic table?
Detailed Solution for Test: Periodic Classification Of Elements (Medium) - Question 1
Test: Periodic Classification Of Elements (Medium) - Question 2
Which of the following is not a noble gas ?
Detailed Solution for Test: Periodic Classification Of Elements (Medium) - Question 2
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Test: Periodic Classification Of Elements (Medium) - Question 3
The element placed with cerium, in the table formed by Newlands' was:
Detailed Solution for Test: Periodic Classification Of Elements (Medium) - Question 3
Test: Periodic Classification Of Elements (Medium) - Question 4
What were the limitations of Mendeleev's periodic table?
Test: Periodic Classification Of Elements (Medium) - Question 5
Element 'X' has twelve protons in its nucleus.To which group of the periodic table it will belong?
Test: Periodic Classification Of Elements (Medium) - Question 6
Name the element that first shows some visible metallic properties at room temperature.
Detailed Solution for Test: Periodic Classification Of Elements (Medium) - Question 6
Test: Periodic Classification Of Elements (Medium) - Question 7
Which among the following elements is metalloid?
Detailed Solution for Test: Periodic Classification Of Elements (Medium) - Question 7
|
5 videos|292 docs|59 tests
|
Information about Test: Periodic Classification Of Elements (Medium) Page
In this test you can find the Exam questions for Test: Periodic Classification Of Elements (Medium) solved & explained in the simplest way possible.
Besides giving Questions and answers for Test: Periodic Classification Of Elements (Medium), EduRev gives you an ample number of Online tests for practice
|
5 videos|292 docs|59 tests
|
Download as PDF

















