Test: Periodic Classification of Elements (Hard) - Class 10 MCQ
20 Questions MCQ Test - Test: Periodic Classification of Elements (Hard)
The elements A, B, and C belong to group 2, 14, and 16 respectively, of the periodic table. Which of the two elements will form covalent bonds?
Which of the following does not decrease while moving down the group of the periodic table?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
An element X belongs to the 3rd period and 1st group of the periodic table. What is the number of valence electrons in its atom?
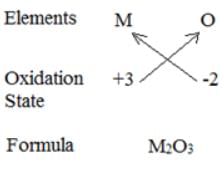
An element M is in group 13th of the periodic table, the formula for its oxide is
Consider The Following Statements And Choose The Incorrect One:
Arrange The Following Elements In The Order Of Their Increasing Nonmetallic Character: Li, O, C, Be, F
Which Of The Following Are The Characteristics Of Isotopes Of An Element?
Which One Of The Following Does Not Increase While Moving Down The Group Of The Periodic Table?
Which Of The Following Is The Outermost Shell For Elements Of Period 2?
Where Would You Locate The Element With Electronic Configuration 2, 8 In The Modern Periodic Table?
The Elements A, B, C, D, and E Have Atomic Number 9, 11, 17, 12, and 13 Respectively. Which Pair Of Elements Belongs To The Same Group?
In A Science Quiz Competition, Simran Are Asked A Question Where She Had To Choose The Statement Which Was/Were Incorrect?
According To Mendeleev’s Periodic Law, The Elements Were Arranged In The Periodic Table In The Order Of
Upto Which Element, The Law Of Octaves Was Found To Be Applicable.
Read The Following Sentences Carefully, And Choose The Incorrect One(S):
Which Of The Following Gives The Correct Increasing Order Of The Atomic Radii Of O, F, and N?
The law of octaves is given by ___.
Which of the following elements will form an acidic oxide?
The element with atomic number 14 is hard and forms acidic oxide and a covalent halide. To which of the following categories does the element belong?
Which of the following set of elements is written in order of their increasing metallic character?