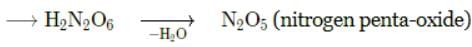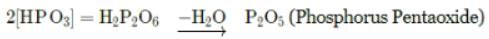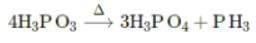Test: Phosphorus Halides & Oxoacids of Phosphorus - NEET MCQ
10 Questions MCQ Test - Test: Phosphorus Halides & Oxoacids of Phosphorus
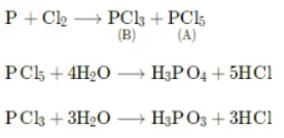
A translucent white waxy solid (A) reacts with excess of chlorine to give a yellowish white powder (B). (B) reacts with organic compounds containing -OH group converting them into chloro derivatives. (B) on hydrolysis gives (C) and is finally converted to phosphoric acid. (A), (B) and (C) are?
Which of the following is not correctly matched?
On reaction with Cl2, phosphorus forms two types of halides 'A' and 'B'. Halide 'A' is yellowish-white powder but halide 'B' is colourless oily liquid. What would be the hydrolysis products of 'A' and 'B' respectively?
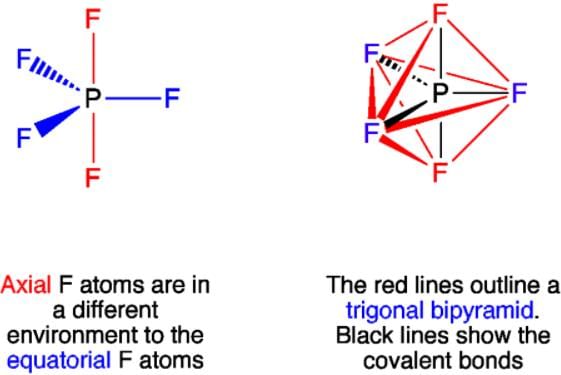
Which of the following statements is not correct about the structure of PCl5?
Why are all P-F bonds in PF5 are not equivalent?
Match the column I with column II and mark the appropriate choice.

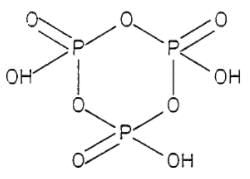
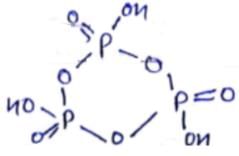
How many P - O - P bonds appear in cyclic metaphosphoric acid?
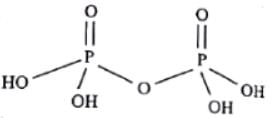
Which of the following is a tetrabasic acid?
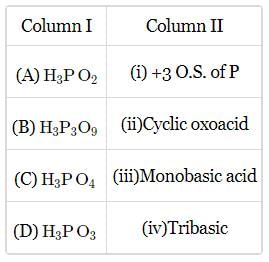
Match the column I and column II and mark the appropriate choice.
Phosphorous acid on heating gives the following products:
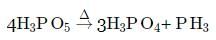
The above reaction is an example of