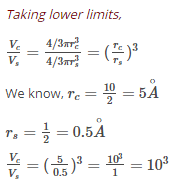Test Previous Year Questions (Level - 1): Surface Chemistry - Class 12 MCQ
8 Questions MCQ Test - Test Previous Year Questions (Level - 1): Surface Chemistry
Adding alum to turbid water, impurties are removed due to -
[AIEEE-2002]
Which one of the following characteristics is not correct for physical adsorption ?
[AIEEE-2003]
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The volume of a colloidal particle, VC as compared to the volume of a solute particle in a true solution VS, could be –
[AIEEE-2005]
The disperse phase in colloidal iron (III) hydroxide and colloidal gold is positively and negatively charged respectively. Which of the following statements is not correct?
[AIEEE-2005]
In Langmuir's model of adsorption of a gas on a solid surface –
[AIEEE 2006]
Gold numbers of protective colloids A, B, C and D are 0.50, 0.01, 0.10 and 0.005, respectively. The correct order of their protective powers is
[AIEEE 2008]
Which of the following statements is incorrect regarding physissorptions ?
[AIEEE 2009]
The coagulating power of electrolytes having ions Na+, Al3+ and Ba2+ for arsenic sulphide sol increases in the order:
[IIT Mains 2013]