Test: Properties of Gases - 1 - Mechanical Engineering MCQ
10 Questions MCQ Test - Test: Properties of Gases - 1
Two insulated tanks containing ideal gases at different pressures and temperatures are connected to each other arid gases are allowed to mix.The process that occurs can be called
Which of the following parameters does not indicate the departure of real gas from the ideal gas behaviour?
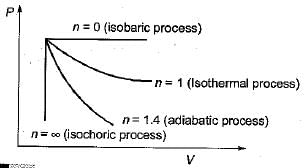
If value of n is infinitely large in a polytropic process P Vn= C, then the process is known as constant
Match List-I (Isotherms) with List-ll (Value of T/TC) pertaining to the generalized compressibility charts in terms of reduced properties and select the correct answer.

In the Van der Waal’s equation, the unit of constant a is
The temperature at which a real gas obeys the ideal gas laws over a wide range of pressure is called
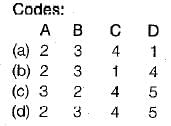
For an ideal gas, the slope of the constant- volume line in the T-S diagram is
The work done in a reversible adiabatic process from state 1 to state 2 is given by



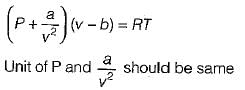
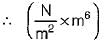 is unit of a, so unit of a is Nm4
is unit of a, so unit of a is Nm4