Test: Properties of Pure Substances - 2 - Mechanical Engineering MCQ
9 Questions MCQ Test - Test: Properties of Pure Substances - 2
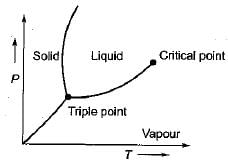
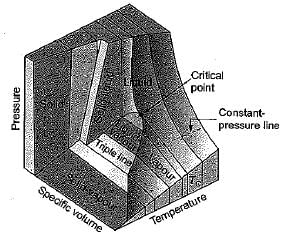
On a P-V-T surface, the triple point and critical point are seen as respectively
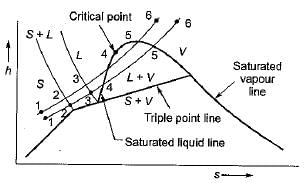
In the mollier diagram for steam, dryness fraction lines converge at
For a mixture of solid, liquid and vapour phases of a pure substance in equilibrium, what is the number of independent intrinsic properties needed ?
Which one of the following represents the condensation of a mixture of saturated liquid and saturated vapour on the enthalpy-entropy diagram
Which of the following are pure substances?
1. Gaseous air
2. A mixture of gaseous air and liquid water
3. A mixture of liquid water and water vapour
4. A mixture of gaseous air and oil
Change of state, e.g., freezing, melting, evaporation and condensation, is an
The INCORRECT statement about the characteristics of critical point of a pure substance is that