Test: Reaction Mechanism Level - 6 - Chemistry MCQ
24 Questions MCQ Test - Test: Reaction Mechanism Level - 6
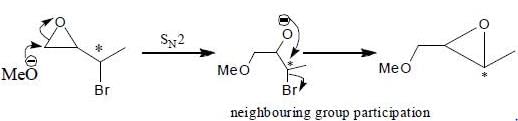
Which of the following statement(s) is/are true about the reaction given below:
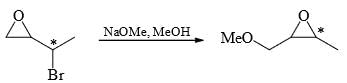
(1) it involves a carbocation intermediate
(2) rearrangement is due to SN1 reaction mechanism.
(3) it proceeds via a concerted SN2 pathway
(4) it involves neighbouring group participation.
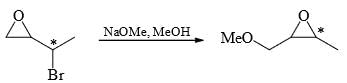
(2) rearrangement is due to SN1 reaction mechanism.
(3) it proceeds via a concerted SN2 pathway
(4) it involves neighbouring group participation.
Compound(s) that on hydrogenation product(s) optically inactive compound(s) is(are):
When 20 g of a compound (A) (M.F. = C4H10O4) reacts with excess of CH3MgBr, 14.6 L of CH4 is obtained at STP. What is structural formula of (A)?
In the following reaction, the product(s) formed is (are)
The major product(s) of the following reaction is (are):
Which of the following ethers can be synthesized directly by Williamson’s synthesis:
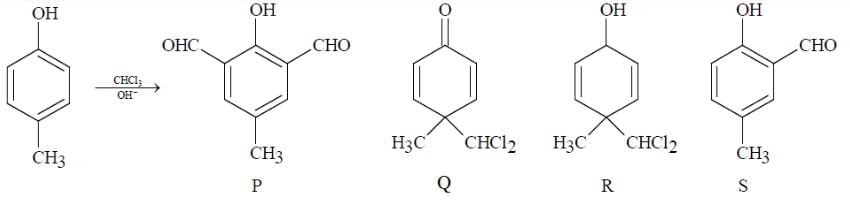
Among P, Q, R and S the aromatic compound(s) is/are:
The correct statement(s) about the following reaction sequence is/are:

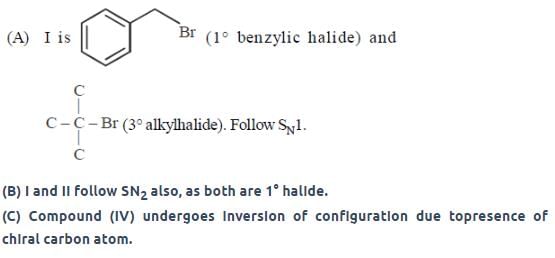
For the following compounds, the correct statement(s) with respect to nucleophile substitution reaction is(are):
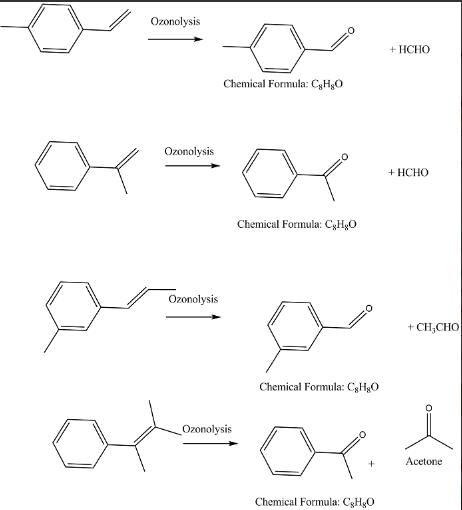
Compounds P and R upon ozonolysis produce Q and S, respectively. The molecular formula of Q and S is C8H8O. Q undergoes Cannizzaro reaction but not Haloform reaction, whereas S undergoes Haloform reaction but no Cannizzaro reaction.

The option(s) with suitable combination of P and R, respectively, is(are)
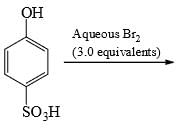
The reactivity of compound Z with different halogens under appropriate conditions is given below:
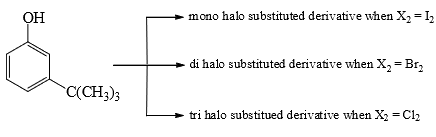
The observed pattern of electrophilic substitution can be explained by:
In the reaction shown below, the major product(s) formed is/are:
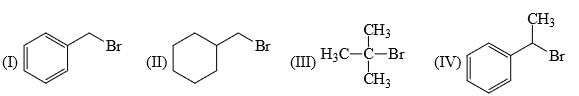
Choose the correct one which will react faster in the SN2 nucleophilic substitution reaction?
Which of the following compound gives even number of Hoffmann’s exhaustive methylation and elimination?
Alcohol on refluxing with Cr2O7 gives ____________
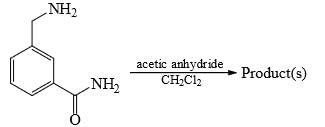
Which of the following compounds will react with R-SH in aqueous solutions between pH 6.5 and 8.5: [TIFR 2012]
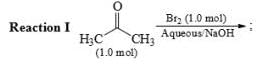
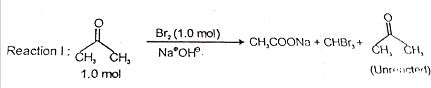
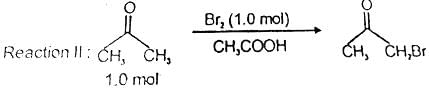
After completion of the reactions (I and II), the organic compound(s) in the reaction mixture is(are):

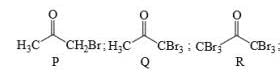
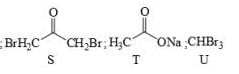
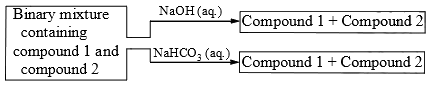
Identify the binary mixture(s) that can be separated into individual compounds, by differential extraction, as shown in the given scheme.
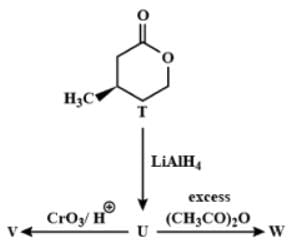
With reference to the scheme given, which of the given statement(s) about T, U, V and W is (are) correct:
Which of the following compounds will react with ethanolic KCN:




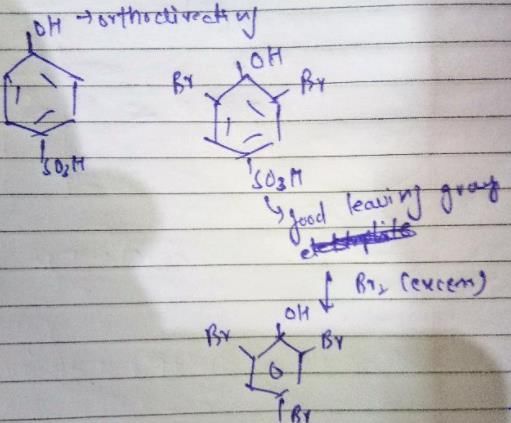
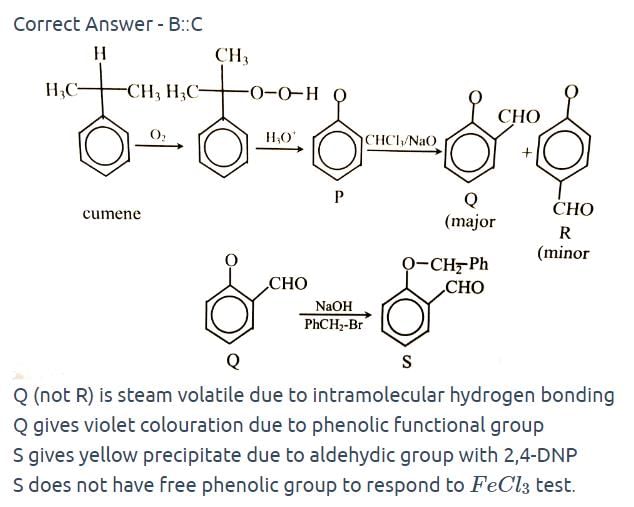

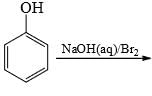 the intermediate(s) is/are:
the intermediate(s) is/are:










