Test: Second Law of Thermodynamics & Entropy - JEE MCQ
20 Questions MCQ Test - Test: Second Law of Thermodynamics & Entropy
Direction (Q. Nos. 1-10) This section contains 10 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. Entropy change for the following reversible process is 1 mole H2O
(l, 1 atm, 100°C )  1 mole H2O (g , 1 atm, 100°C)(ΔHvap = 40850 J mol-1)
1 mole H2O (g , 1 atm, 100°C)(ΔHvap = 40850 J mol-1)
Entropy change when 2 moles of an ideal gas expands reversibly from an initial volume of 1 dm3 to a final volume of 10 dm3 at a constant temperature of 298 K is
3 moles of a diatomic gas are heated from 127° C to 727° C at a constant pressure of 1 atm. Entropy change is (log 2.5 = 0 .4)
10 dm3 of an ideal monoatomic gas at 27° C and 1.01 x 105 Nm-2 pressure are heated at constant pressure to 127°C. Thus entropy change is
Exactly 100 J of heat was transferred reversibly to a block of gold at 25.00° C from a thermal reservoir at 25.01 °C, and then exactly 100 J of heat was absorbed reversibled from the block of gold by a thermal reservoir at 24.99° C. Thus entropy change of the system is
Given
I. C (diamond) + O2(g) → CO2(g) ; ΔH° = - 91.0 kcdl mol-1
II. C(graphite) + O2(g) → CO2(g) ; ΔH° = - 94.0 kcal mol-1
Q. At 298 K, 2.4 kg of carbon (diamond) is converted into graphite form. Thus, entropy change is
Consider a reversible isentropic expansion of 1.0 mole of an ideal monoatomic gas from 25°C to 75°C. If the initial pressure was 1.0 bar, final pressure is
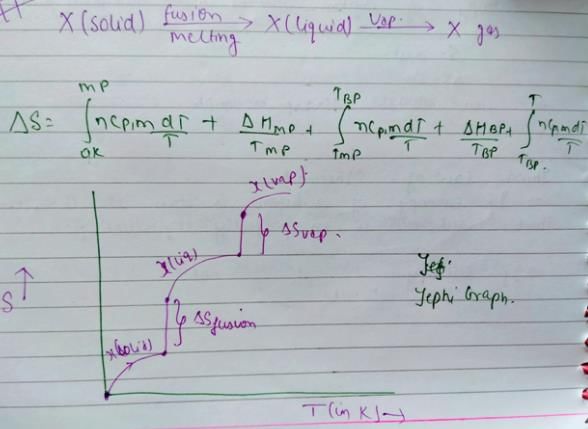
Consider the following figure representing the increase in entropy of a substance from absolute zero to its gaseous state at some temperature
Q. ΔS° (fusion) and ΔS° (vaporisation) are respectively indicated by
ΔHvap = 30 kJ mol-1 and ΔSvap = 75 J mol-1 K-1. Thus, temperature of the vapour at 1 atm is
[IIT JEE 2004]
For the process, and 1 atmosphere pressure, the correct choice is
[JEE Advanced 2014]
Direction (Q. Nos. 11-14) This section contains 4 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THANT ONE is correct.
Q. For an ideal gas, consider only (p -V) work in going from initial state X to the final state Z. The final state Z can be reached either of the two paths shown in the figure. Which of the following choice (s) is (are) correct?
(Take ΔS as change in entropy and W as work done)
[IIT JEE 2012]
Benzene and naphthalene form an ideal solution at room temperature. For this process, the true statement(s) is (are)
[JEE Advanced 2013]
Consider the following process
Select correct choices(s)
In which of the following cases, entropy of I is larger than that of II?
Direction (Q. Nos. 15 and 18) This section contains 2 paragraphs, each describing theory, experiments, data etc. Four questions related to the paragraphs have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d)
Passage I
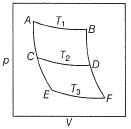
Consider a series of isotherms and adiabates as shown
AB, CD and EF are isotherms.
AC, CE, BD and D F are adiabates.
Q.
Passage I
Consider a series of isotherms and adiabates as shown
AB, CD and EF are isotherms.
AC, CE, BD and D F are adiabates.
Q. Select incorrect relationship
Passage II
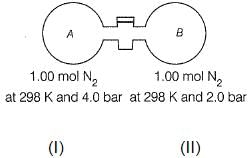
The stopcock connecting A and B is of negligible volume. Stopcock is opened and gases are allowed to mix isothermally.
Q. Final pressure set up is
Passage II
The stopcock connecting A and B is of negligible volume. Stopcock is opened and gases are allowed to mix isothermally.
Q. Entropy change for the system is
Direction (Q. Nos. 19 and 20) This section contains 2 questions. Each question, when worked out will result in an integer from 0 fo 9 (both inclusive).
Q. Consider two Carnot engines (1) and (2)
Efficiency η2
Derive the value of η2 η1
A cyclic heat engine operates between a source temperature of 927 oC and a sink temperature of 27 oC. What will be the maximum efficiency of the heat engine?