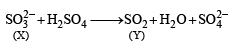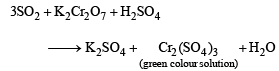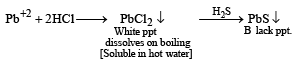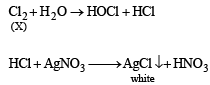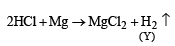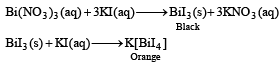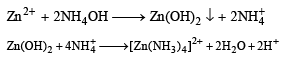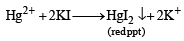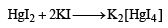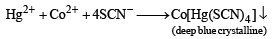Test: Single Correct MCQs: Analytical Chemistry | JEE Advanced - JEE MCQ
13 Questions MCQ Test 35 Years Chapter wise Previous Year Solved Papers for JEE - Test: Single Correct MCQs: Analytical Chemistry | JEE Advanced
The ion that cannot be precipitated by both HCl and H2S is
Which one among the following pairs of ions cannot be separated by H2S in dilute hydrochloric acid? (1986 - 1 Mark)
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
An aqueous solution contains Hg2+, Hg22+, Pb2+ and Cd2+.The addition of HCl (6N) will precipitate : (1995S)
Identify the correct order of solubility of Na2S, CuS and ZnS in aqueous medium (2002S)
An aqueous solution of a substance gives a white precipitate on treatment with dilute hydrochloric acid, which dissolves on heating. When hydrogen sulfide is passed through the hot acidic solution, a black precipitate is obtained. The substance is a (2002S)
A gas 'X' is passed th rough water to form a satur ated solution. The aqueous solution on treatment with silver nitrate gives a white precipitate. The saturated aqueous solution also dissolves magnesium ribbon with evolution of a colourless gas 'Y'. Identify 'X' and 'Y'. (2002S)
[X] + H2SO4 —→ [Y] a colourless gas with irritating smell, [Y] + K2Cr2O7 + H2SO4 —→ green solution. [X] and [Y] are:(2003S)
A solution which is 10-3 M each in Mn2+, Fe2+, Zn2+ and Hg2+ is treated with 10-16 M sulphide ion. If Ksp of MnS, FeS, ZnS and HgS are 10-15, 10-23, 10-20 and 10-54 respectively, which one will precipitate first? (2003S)
A metal nitrate reacts with KI to give a black precipitate which on addition of excess of KI is converted into orange colour solution. The cation of the metal nitrate is (2005S)
A solution when diluted with H2O and boiled, gives a white precipitate. On addition of excess NH4Cl/NH4OH, the volume of precipitate decreases leaving behind a white gelatinous precipitate. Identify the precipitate which disolves in NH4OH/NH4Cl (2006 - 3M, –1)
A solution of a metal ion when treated with KI gives a red precipitate which dissolves in excess KI to give a colourless solution. Moreover, the solution of metal ion on treatment with a solution of cobalt (II) thiocyanate gives rise to a deep blue crystalline precipitate. The metal ion is (2007)
Passing H2S gas into a mixture of Mn2+, Ni2+, Cu2+ and Hg2+ ions in an acidified aqueous solution precipitates (2011)
Upon treatment with ammoniacal H2S, the metalion that precipitates as a sulfide is (JEE Adv. 2013)
|
347 docs|185 tests
|
|
347 docs|185 tests
|