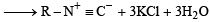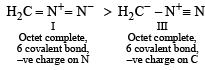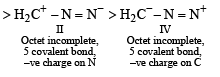Test: Single Correct MCQs: Compounds Containing Nitrogen | JEE Advanced - JEE MCQ
23 Questions MCQ Test 35 Years Chapter wise Previous Year Solved Papers for JEE - Test: Single Correct MCQs: Compounds Containing Nitrogen | JEE Advanced
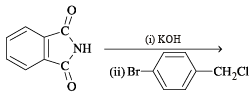
The compound which on reaction with aqueous nitrous acid at low temperature produces an oily nitrosamine is
Acetamide is treated separately with the following reagents.
Which one of these would give methylamine?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Carbylamine test is performed in alcoholic KOH by heating a mixture of :
The compound that is most reactive towards electrophilic nitration is :
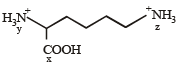
If two compounds have the same empirical formula but different molecular fomulae they must have
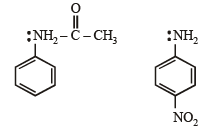
Amongst the following, the most basic compound is :
The formation of cyanohydrin from a ketone is an example of :
Butanonitrile may be prepared by heating :
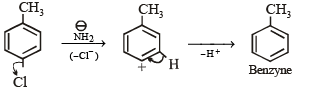
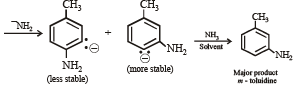
In the reaction p-chlorotoluene with KNH2 in liq. NH3,the major product is:
The most unlikely representation of resonance structures of p-nitrophenoxide ion is
Among the following, the strongest base is
The correct order of basicities of the following compounds is
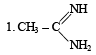
2. CH3 - CH2 - NH2
3. (CH3)2NH
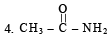
Compound 'A' (molecular formula C3H8O) is treated with acidified potassium dichromate to form a product 'B' (molecular formula C3H6O). 'B' forms a shining silver mirror on warming with ammonical silver nitrate. 'B' when treated with an aqueous solution of H2NCONHNH2.HCl and sodium acetate gives a product 'C'. Identify the structure of 'C'.
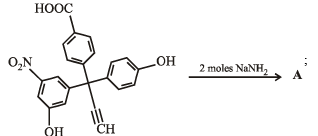
The product A will be
Benzamide on reaction with POCl3 gives
The major product obtained when Br2/Fe is treated with
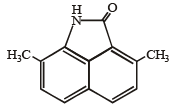
In the compound given below the correct order of the acidity of the positions X, Y and Z is
When benzenesulfonic acid and p-nitrophenol are treated with NaHCO3, the gases released respectively are
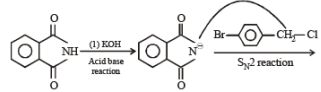
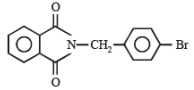
In the following reaction,
CH3 NH2 + CHCl3+ KOH → Nitrogen containing compound + KCl + H2O.
The nitrogen containing compound is
The correct stability order of the following resonance structures is
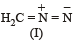
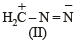
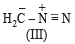
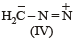
The major product of the following reaction is
Amongst the compounds given, the one that would form a brilliant colored dye on treatment with NaNO2 in dil. HCl followed by addition to an alkaline solution of β-naphthol is
|
347 docs|185 tests
|
|
347 docs|185 tests
|


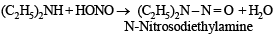
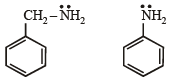
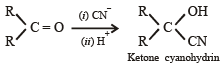
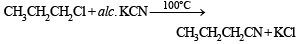
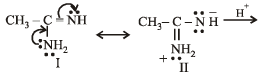
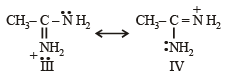
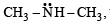 the availability of electron pair increases due to the +I effect of two CH3 groups while in CH3CH2NH2, +I effect of only one ethyl group is operative. In
the availability of electron pair increases due to the +I effect of two CH3 groups while in CH3CH2NH2, +I effect of only one ethyl group is operative. In 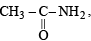 the electron availability on nitrogen decreases due to resonance as shown below
the electron availability on nitrogen decreases due to resonance as shown below
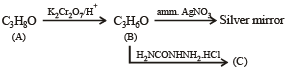
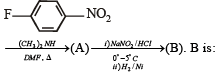
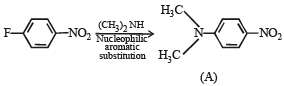
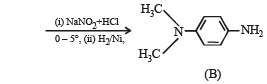
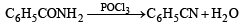
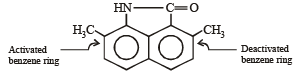
 group is a deactivating group.
group is a deactivating group.