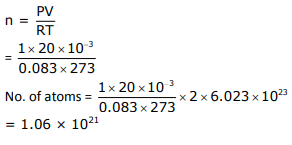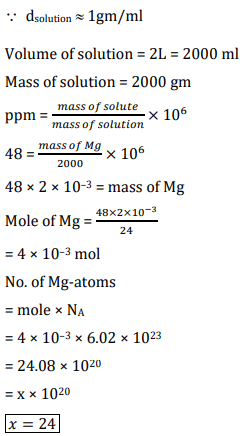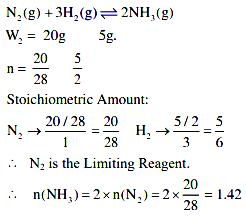Test: Some Basic Concepts of Chemistry (April 9 ) - JEE MCQ
15 Questions MCQ Test Daily Test for JEE Preparation - Test: Some Basic Concepts of Chemistry (April 9 )
The number of N atoms in 681 g of C7H5N3O6 is x × 1021. The value of x is ______. (NA = 6.02 × 1023 mol-1) (Nearest Integer)
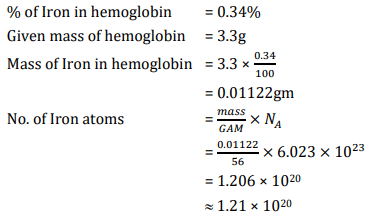
Hemoglobin contains 0.34% of iron by mass. The number of Fe atoms in 3.3 g of hemoglobin is: (Given: Atomic mass of Fe is 56 u, NA is 6.022 × 1023 mol-1)
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
A 2.0 g sample containing MnO2 is treated with HCl liberating Cl2. The Cl2 gas is passed into a solution of KI and 60.0 mL of 0.1 M Na2S2O3 is required to titrate the liberated iodine. The percentage of MnO2 in the sample is ____________ . (Nearest integer)
[Atomic masses (in u) Mn = 55; Cl = 35.5; O = 16, I = 127, Na = 23, K = 39, S = 32]
[Atomic masses (in u) Mn = 55; Cl = 35.5; O = 16, I = 127, Na = 23, K = 39, S = 32]
The number of chlorine atoms in 20 mL of chlorine gas at STP is _____ 1021.
(Round off to the nearest integer)
[Assume chlorine is an ideal gas at STP, R = 0.083 L bar mol-1 K-1, NA = 6.023 × 1023]
On complete combustion of 0.492 g of an organic compound containing C, H and O, 0.7938 g of CO2 and 0.4428 g of H2O was produced. The % composition of oxygen in the compound is ________.(In integer)
Amongst the following statements, that which was not proposed by Dalton was:
Chlorophyll extracted from the crushed green leaves was dissolved in water to make 2 L solution of Mg of concentration 48 ppm. The number of atoms of Mg in this solution is x × 1020 atoms. The value of x is _________. (Nearest Integer)
(Given: Atomic mass of Mg is 24 g mol-1, NA = 6.02 × 1023 mol-1)
Two elements A and B form 0.15 moles of A2B and AB3 type compounds. If both A2B and AB3 weigh equally, then the atomic weight of A is ________ times of atomic weight of B. (In integer)
The mole fraction of a solute in a 100 molal aqueous solution _______ × 10-2.
(Round off to the nearest integer)
[Given: Atomic masses: H: 1.0 u, O: 16.0 u]
The complete combustion of 0.492 g of an organic compound containing 'C', 'H' and 'O' gives 0.793 g of CO2 and 0.442 g of H2O. The percentage of oxygen composition in the organic compound is __________. (Nearest integer)
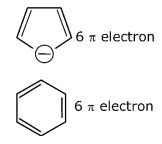
Among the following, the aromatic compounds are:
(A)
(B)
(C)
(D)
Choose the correct answer from the following options:
Two solutions, A and B, each of 100 L was made by dissolving 4 g of NaOH and 9.8 g of H2SO4 in water, respectively. The pH of the resultant solution obtained from mixing 40 L of solution A and 10 L of solution B is ________.
[Answer upto two decimal places]
Complete combustion of 750 g of an organic compound provides 420 g of CO2 and 210 g of H2O. The percentage composition of carbon and hydrogen in organic compound is 15.3 and ________, respectively. (Round off to the nearest integer)

Consider the above reaction, the limiting reagent of the reaction and the number of moles of NH3 formed respectively are:
56 L of nitrogen gas is mixed with excess of hydrogen gas and it is found that 20 L of ammonia gas is produced. The volume of unused nitrogen gas is found to be ____ L.
|
360 tests
|