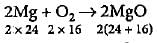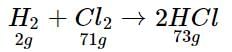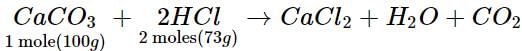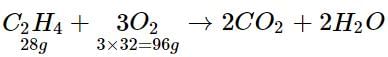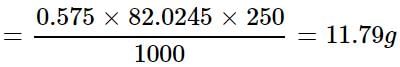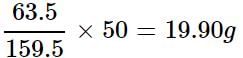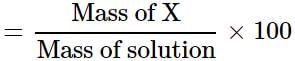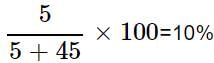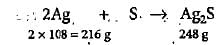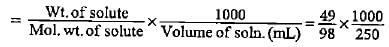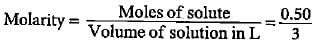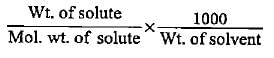Test: Stoichiometry and Stoichiometric Calculations - NEET MCQ
20 Questions MCQ Test - Test: Stoichiometry and Stoichiometric Calculations
1 g of Mg is burnt in a closed vessel containing 0.5 g of O2. Which reactant is limiting reagent and how much of the excess reactant will be left?
In a reaction container, 100 g of h2 and 100 g of CI2 are mixed for the formation of HCl gas. What is the limiting reagent and how much HCl is formed in the reaction?
If 40 g of CaCO3 is treated with 40 g of HCl, which of the reactants will act as limiting reagent?
The weight of AgCl precipitated when a solution containing 5.85 g of NaCl is added to a solution containing 3.4 g of AgNO3 is
How much oxygen is required for complete combustion of 560 g of ethene?
What is the mass percent of oxygen in ethanol?
How much mass of sodium acetate is required to make 250 mL of 0.575 molar aqueous solution?
How much copper is present in 50 g of CuSO4?
A solution is prepared by adding 5 g of a solute 'X' to 45 g of solvent 'Y'. What is the mass per cent of the solute 'X'?
An impure sample of silver (1.5 g) is heated with S to form 0.124 g of Ag2S. What was the per cent yield of Ag2S?
2.82 g of glucose is dissolved in 30 g of water. The mole fraction of glucose in the solution is
What volume of water is to be added to 100 cm3 of 0.5 M NaOH solution to make it 0.1 M solution?
The final molarity of a solution made by mixing 50 mL of 0.5 M HCl, 150 mL of 0.25 M HCl and water to make the volume 250 mL is
A solution is made by dissolving 49 g of H2SO4 in 250 mL of water. The molarity of the solution prepared is
What is the concentration of copper sulphate (in mol L-1) if 80 g of it is dissolved in enough water to make a final volume of 3 L?
4.28 g of NaOH is dissolved inwater and the solution is made to 250 cc. What will be the molarity of the solution?
What volume of 5 M Na2SO4 must be added to 25 mL of 1 M BaCl2 to produce 10 g of BaSO4?
What will be the molarity of the solution in which 0.365 g of HCl gas is dissolved in 100 mL of solution?
What will be the molality of the solution made by dissolving 10 g of NaOH in 100 g of water?
What will be the molality of chloroform in the water sample which contains 15 ppm chloroform by mass?