Test: Thermodynamic Relations - 1 - Mechanical Engineering MCQ
10 Questions MCQ Test - Test: Thermodynamic Relations - 1
For an isentropic flow along a nozzle, at location A, the static temperature and pressure were measured to be 320 K and 5 bar. What will be the static pressure at the location where the static temperature is 500 K? (Given η = 1.4)
Average coefficient of volume expansion of a substance is denoted by
A gas having a negative Joule-Thomson coefficient ( μj < 0), when throttled, will
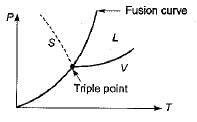
The Clausius-Clapeyron equation gives the slope of curve on
Consider the following statements pertaining to the Clapeyron equation:
1. It is useful in estimating properties like enthalpy from other measurable properties.
2. At a change of phase, it can be used to find the latent heat at a given pressure.
3. It is derived from the relationship
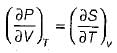
Which of the statements given above are correct?
Which of the following parameter is.the criterion of equilibrium and stability of a system existing at constant volume and constant temperature?
The internal energy of an ideal gas is a function of