Test: Thermodynamics(7 Sep) - JEE MCQ
15 Questions MCQ Test Daily Test for JEE Preparation - Test: Thermodynamics(7 Sep)
When the temperature of a metal wire is increased from 0°C to 10°C, its length increases by 0.02%. The percentage change in its mass density will be closest to:
Starting with the same initial conditions, an ideal gas expands from volume V1 to V2 in three different ways. The work done by the gas is W1 if the process is purely isothermal, W2 if the process is purely adiabatic and W3 if the process is purely isobaric. Then,
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
A leak proof cylinder of length 1 m, made of a metal which has very low coefficient of expansion, is floating vertically in water at 0°C such that its height above the water surface is 20 cm. When the temperature of water is increased to 4°C, the height of the cylinder above the water surface becomes 21 cm. The density of water at T = 4°C relative to the density at T = 0°C is close to: (Answer up to 2 decimal places)
A monoatomic gas at pressure P and volume V is suddenly compressed to one eighth of its original volume. The final pressure at constant entropy will be:
The efficiency of a Carnot's engine, working between steam point and ice point, will be:
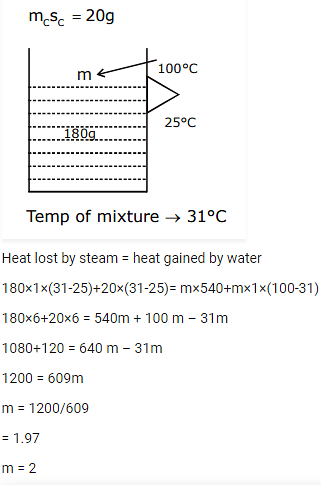
A calorimeter of water equivalent 20 g contains 180 g of water at 25°C. 'm' grams of steam at 100°C is mixed in it till the temperature of the mixture is 31°C. The value of 'm' is close to (Latent heat of water = 540 cal g-1, specific heat of water = 1 cal g-1 °C-1)
The specific heat of water = 4200 J kg-1K-1 and the latent heat of ice = 3.4 × 105 J kg-1. 100 grams of ice at 0°C is placed in 200 g of water at 25°C. The amount of ice that will melt as the temperature of water reaches 0°C is close to (in grams) (Answer upto 1 decimal place)
Three containers C1, C2 and C3 have water at different temperatures. The table below shows the final temperature T when different amounts of water (given in litres) are taken from each container and mixed (assume no loss of heat during the process).

The value of (in °C, to the nearest integer) is _______.
7 moles of certain monoatomic ideal gas undergoes a temperature increase of 40 K at constant pressure. The increase in the internal energy of the gas in this process is
(Given R = 8.3 JK-1 mol-1)
A Carnot engine operates between two reservoirs of temperatures 900 K and 300 K. The engine performs 1,200 J of work per cycle. The heat energy (in J) delivered by the engine to the low temperature reservoir, in a cycle, is (in integers)
For an adiabatic expansion of an ideal gas, the fractional change in its pressure is equal to (where is the ratio of specific heats):
A Carnot's engine working between 400 K and 800 K has a work output of 1200 J per cycle. The amount of heat energy supplied to the engine from the source in each cycle is:
The amount of heat needed to raise the temperature of 4 moles of a rigid diatomic gas from 0°C to 50°C when no work is done is ______. (R is the universal gas constant.)
A balloon filled with helium (32°C and 1.7 atm.) bursts. Immediately afterwards the expansion of helium can be considered as:
A block of ice of mass 120 g at temperature 0°C is put in 300 gm of water at 25°C. The x g of ice melts as the temperature of the water reaches 0°C. The value of x is (in integers)
[Use: Specific heat capacity of water = 4,200 Jkg-1K-1, Latent heat of ice = 3.5 × 105 Jkg-1]
|
360 tests
|



 ... (i)
... (i) = 2 × 10-4
= 2 × 10-4 


 = 2 × 10-5
= 2 × 10-5 × 100 = 6 × 10-5 × 10 × 100
× 100 = 6 × 10-5 × 10 × 100








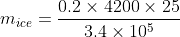
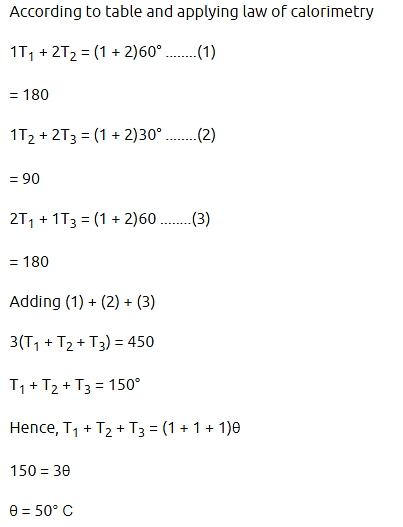
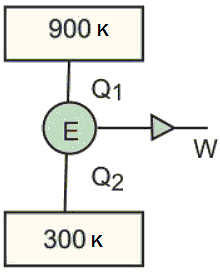



 Q1 = 1,800 J
Q1 = 1,800 J Q2 = Q1 - W = 1,800 - 1,200 = 600 J
Q2 = Q1 - W = 1,800 - 1,200 = 600 J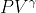 = constant
= constant

 (
(


 = 0
= 0

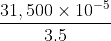 = 9,000 × 10-5
= 9,000 × 10-5














