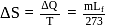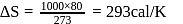Test: Third law, Heat transfer - JEE MCQ
10 Questions MCQ Test Chemistry for JEE Main & Advanced - Test: Third law, Heat transfer
Fixed mass of an ideal gas contained in a 24.63 L sealed rigid vessel at 1 atm is heated from −73∘C to 27∘C. Calculate change in gibb's energy if entropy of gas is a function of temperature as S = 2 + 10−2 T(J/K):(Use 1 atm L = 0.1 kJ)
For the reaction taking place at certain temperature 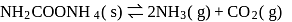 if equilibrium pressure is
if equilibrium pressure is 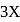 bar then
bar then 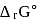 would be
would be
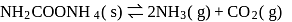 if equilibrium pressure is
if equilibrium pressure is 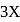 bar then
bar then 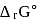 would be
would be| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Standard Gibb's free energy change for isomerization reaction cis-2-pentene ⇌ trans − 2 -pentene is −3.67 kJ/mol at 400 K. If more trans-2pentene is added to the reaction vessel, then
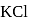 in water is endothermic yet it dissolves in water spontaneously. Which one of the following best explains this behaviour?
in water is endothermic yet it dissolves in water spontaneously. Which one of the following best explains this behaviour?
What is the free energy change for the conversion of 1 mole of water into steam at 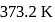 . The heat of vaporization
. The heat of vaporization 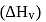 of water of
of water of 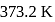 is
is  . The entropy change is
. The entropy change is 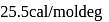 .
.
The favourable conditions for a spontaneous reaction are
Find the value of the equilibrium constant (K) of a reaction at 300 K, when standard Gibbs free energy change is −25 kJ mol−1 ? (Consider R = 8.33Jmol−1 K−1)
In a thermodynamic process, fixed mass of a gas is changed in such a manner that the gas release 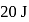 of heat and
of heat and  of work was done on the gas. If the initial internal energy of the gas was
of work was done on the gas. If the initial internal energy of the gas was 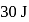 , the final internal energy will be
, the final internal energy will be
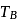 and freezing point is
and freezing point is 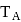 , then correct variation shown by, graph between entropy change and temperature is
, then correct variation shown by, graph between entropy change and temperature is
When 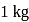 of ice at
of ice at 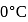 melts to water at
melts to water at 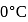 , the resulting change in its entropy, taking latent heat of ice to be
, the resulting change in its entropy, taking latent heat of ice to be  , is
, is
|
352 videos|596 docs|309 tests
|
|
352 videos|596 docs|309 tests
|


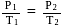
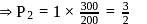
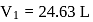
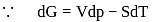
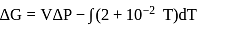
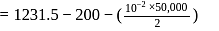
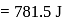
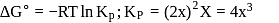
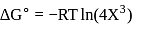
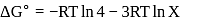
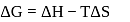
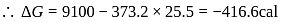
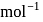
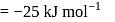
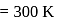
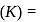 ?
?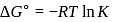
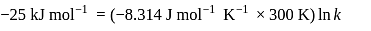
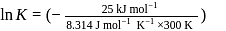
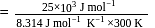
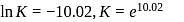
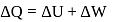
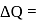 heat absorbed by gas
heat absorbed by gas 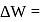 work done by gas.
work done by gas.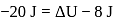
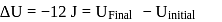
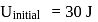
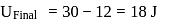
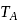 and
and 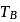 represent the same temperature. Hence
represent the same temperature. Hence 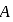 is a correct choice.
is a correct choice.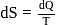 or
or