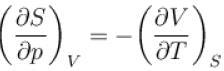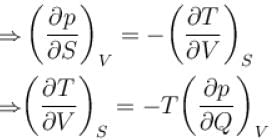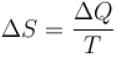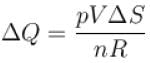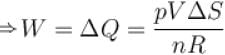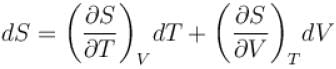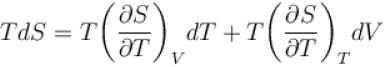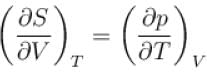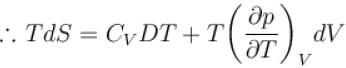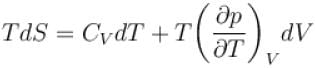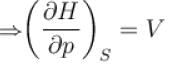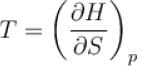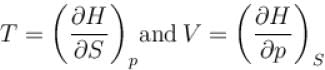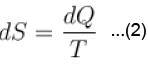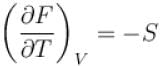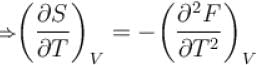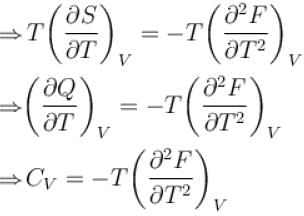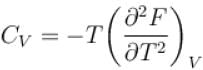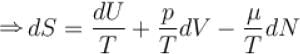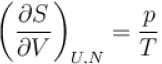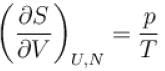Thermodynamic Potential MCQ Level – 1 - Physics MCQ
10 Questions MCQ Test Topic wise Tests for IIT JAM Physics - Thermodynamic Potential MCQ Level – 1
The thermodynamic relation expressing the change at temperature with volume at constant entropy is
Select one:
Select one:
A sample of an ideal gas with initial pressure p and volume V is taken through an isothermal expansion proceed during which the change in entropy is found to be ΔS. The universal gas constant is R. Then, the work done by the gas is given by
Select one:
Select one:
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The Gibb’s potential is defined as :
Select one:
Select one:
The thermodynamical relation expressing TdS equation.
Select one:
The thermodynamical potential enthalpy H = U + pV, then which of the following relation hold true?
Select one:
The combined form of first and second law of thermodynamics is given by (p = pressure, V = volume, T = temperature, U = internal energy, S = entropy, Q = quantity of heat)
Select one:
Helmholtz free energy in given as F = U – Ts then which of the relations hold true?
Select one:
The Gibb’s function G in thermodynamics is defined as
G = H – TS
In an isothermal, isobaric, reversible process, G
(where H = Enthalpy, T = Temperature, S = Entropy)
Select one:
Gibb’s thermodynamical potential can be represented as G = H – TS. Which relation hold true?
[Where H = Enthalpy, S = Entropy]
Select one:
The enthalpy of unit mass for any system is
Select one: