Thermodynamics (Chapter Test - Non Medical) - Class 12 MCQ
20 Questions MCQ Test - Thermodynamics (Chapter Test - Non Medical)
For the reaction, A →B,ΔH = +24kJ /mole
For the reaction, B → C,ΔH = -18kJ /mole
The decreasing order of enthalpy of A, B, C follows the
order
For the reaction, B → C,ΔH = -18kJ /mole
The decreasing order of enthalpy of A, B, C follows the
order
Which of the following equations has/have enthalpy changes equal to ΔHcomb C ?
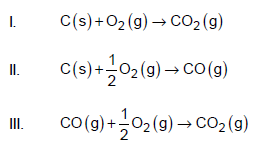
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
(ΔH- ΔU) for the fomation of carbon monoxide (CO) from its element at 298 K is (R = 8.314 J K—1 mol—1)
An ideal gas occupying a volume of 2dm3 at a pressure of 5 bar undergoes isothermal and irreversible
expansion against external pressure of 1 bar. The final volume of the system and the work involved in the
process is
When 1.8 g of steam at the normal boiling point of water is converted into water at the same temperature,
enthalpy and entropy changes respectively will be
The enthalpy change for the hydration,
–71. 50 k cal mol–1. If enthalpy of vaporisation of water is 10.5 k cal mol–1 at 25°C, what would be enthalpy change for the hydration,
An ideal gas expands in volume from 1 x 10—3 m3 to 1 x 10—2 m3 at 300 K against a constant pressure of 1 x 105 N m2. The work done is
How many calories are required to heat 40 gram of argon from 40 to 1000C at constant volume ?
(R = 2 cal/mol K)
The enthalpy of vaporisation of liquid water using data
The enthalpy change for a given reaction at 298 K is –x J mol–1. For the reaction to be spontaneous at 298 K, the entropy change at that temperature,
If all the reactants and the products in a reaction are in their standard states of unit molar concentration, then which is true of the following ?
Given that and
. The standard entropy change in the formation of 1 mole of HCl(g) from H2(g) and Cl2(g) will be
An ice cube at 0.000C is placed in 200 g of distilled water at 250C. The final temperature after the ice is
completely melted is 50C. What is the mass of the ice cube ?
Heat absorbed by a system in going through a cyclic process shown in figure is
Predict the sign of DS for each of the following processes, which occur at constant temperature
I. The volume of 2 mol of O2(g) increases from 44 L to 54 L.
II. The pressure of 2 mol of O2(g) increases from 1 atm to 1.2 atm.
The standard free energy change of the reaction
is about
The value of of the reaction,
at 300 K is (Given : log 0.2 = –0.699)
Which of the following expressions is not correct ?
Ques 19 – 20 are based on the following information 298 K
Q.
The standard enthalpy change of the above reaction is
Q.
The standard entropy change of the reaction is

















