WBJEE Previous Year - 2013 - JEE MCQ
30 Questions MCQ Test - WBJEE Previous Year - 2013
The equation of state of a gas is given by 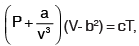 where P, V, T are pressure, volume and temperature respectively, and a, b, c are constants. The dimensions of a and b are respectively
where P, V, T are pressure, volume and temperature respectively, and a, b, c are constants. The dimensions of a and b are respectively
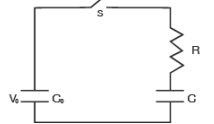
A capacitor of capacitance C0 is charged to a potential V0 and is connected with another capacitor of capacitance C as shown. After closing the switch S, the common potential across the two capacitors becomes V. The capacitance C is given by
The r.m.s. speed of the molecules of a gas at 100°C is ν. The temperature at which the r.m.s. speed will be √3ν is
As shown in the figure below, a charge +2C is situated at the origin O and another charge +5C is on the x-axis at the point A. The later charge from the point A is then brought to a point B on the y-axis. The work done is
A frictionless piston-cylinder based enclosure contains some amount of gas at a pressure of 400 kPa. Then heat is transferred to the gas at constant pressure in a quasi-static process. The piston moves up slowly through a height of 10cm. If the piston has a cross-section area of 0.3 m2, the work done by the gas in this process is
An electric cell of e.m.f. E is connected across a copper wire of diameter d and length l. The drift velocity of electrons in the wire is vd. If the length of the wire is changed to 2l, the new drift velocity of electrons in the copper wire will be
A NOR gate and a NAND gate are connected as shown in the figure. Two different sets of inputs are given to this set up. In the first case, the input to the gates are A=0, B=0, C=0. In the second case, the inputs are A=1, B=0, C=1. The output D in the first case and second case respectively are
A bar magnet has a magnetic moment of 200 A.m2. The magnet is suspended in a magnetic field of 0.30 NA–1 m–1. The torque required to rotate the magnet from its equilibrium position through an angle of 30°, will be
Two soap bubbles of radii r and 2r are connected by a capillary tube-valve arrangement as shown in the diagram. The valve is now opened. Then which one of the following will result:
An ideal mono-atomic gas of given mass is heated at constant pressure. In this process, the fraction of supplied heat energy used for the increase of the internal energy of the gas is
The velocity of a car travelling on a straight road is 36 kmh–1 at an instant of time. Now travelling with uniform acceleration for 10 s, the velocity becomes exactly double. If the wheel radius of the car is 25 cm, then which of the following numbers is the closest to the number of revolutions that the wheel makes during this 10 s?
Two glass prisms P1 and P2 are to be combined together to produce dispersion without deviation. The angles of the prisms P1 and P2 are selected as 4° and 3° respectively. If the refractive index of prism P1 is 1.54, then that of P2 will be
The ionization energy of the hydrogen atom is 13.6 eV. The potential energy of the electron in n = 2 state of hydrogen atom is
Water is flowing in streamline motion through a horizontal tube. The pressure at a point in the tube is p where the velocity of flow is v. At another point, where the pressure is p/2, the velocity of flow is [density of water = ρ]
In the electrical circuit shown in figure, the current through the 4Ω resistor is
A wire of initial length L and radius r is stretched by a length l. Another wire of same material but with initial length 2L and radius 2r is stretched by a length 2l. The ratio of the stored elastic energy per unit volume in the first and second wire is,
A current of 1 A is flowing along positive x-axis through a straight wire of length 0.5 m placed in a region of a magnetic field given by B =(2ˆi+ 4ˆj) T. The magnitude and the direction of the force experienced by the wire respectively are
Two spheres of the same material, but of radii R and 3R are allowed to fall vertically downwards through a liquid of density σ. The ratio of their terminal velocities is
S1 and S2 are the two coherent point sources of light located in the xy-plane at points (0,0) and (0,3λ) respectively.Here λ is the wavelength of light. At which one of the following points (given as coordinates), the intensity of interference will be maximum?
An alpha particle (4He)has a mass of 4.00300 amu. A proton has mass of 1.00783 amu and a neutron has mass of 1.00867 amu respectively. The binding energy of alpha particle estimated from these data is the closest to
Four small objects each of mass m are fixed at the corners of a rectangular wire-frame of negligible mass and of sides a and b (a > b). If the wire frame is now rotated about an axis passing along the side of length b, then the moment of inertia of the system for this axis of rotation is
The equivalent resistance between the points a and b of the electrical network shown in the figure is
The de Broglie wavelength of an electron (mass = 1 × 10–30 kg, charge = 1.6 × 10-19 C) with a kinetic energy of 200 eV is (Planck’s constant = 6.6 × 10–34 J s)
An object placed at a distance of 16 cm from a convex lens produces an image of magnification m (m > 1). If the object is moved towards the lens by 8 cm then again an image of magnification m is obtained. The numerical value of the focal length of the lens is
The number of atoms of a radioactive substance of half-life T is N0 at t = 0. The time necessary to decay from N0/2 atoms to N0/10 atoms will be
A travelling acoustic wave of frequency 500 Hz is moving along the positive x-direction with a velocity of 300 ms–1. The phase difference between two points x1 and x2 is 60º. Then the minimum separation between the two pints is
A mass M at rest is broken into two pieces having masses m and (M-m). The two masses are then separated by a distance r. The gravitational force between them will be the maximum when the ratio of the masses [m:(M-m)] of the two parts is
A shell of mass 5M, acted upon by no external force and initially at rest, bursts into three fragments of masses M, 2M and 2M respectively. The first two fragments move in opposite directions with velocities of magnitudes 2V and V respectively. The third fragment will
A bullet of mass m travelling with a speed v hits a block of mass M initially at rest and gets embedded in it. The combined system is free to move and there is no other force acting on the system. The heat generated in the process will be
A particle moves along X-axis and its displacement at any time is given by x(t) = 2t3 – 3t2 + 4t in SI units. The velocity of the particle when its acceleration is zero, is



















