XL Life Science- 2013 GATE Paper (Practice Test) - GATE MCQ
30 Questions MCQ Test - XL Life Science- 2013 GATE Paper (Practice Test)
Q. No. 1 – 5 Carry One Mark Each
Q.
If 3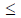 X
X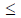 5 and 8
5 and 8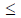 Y
Y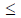 11 then which of the following options is TRUE?
11 then which of the following options is TRUE?
The Headmaster to speak to you.
Which of the following options is incorrect to complete the above sentence?
Which of the following options is incorrect to complete the above sentence?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Mahatama Gandhi was known for his humility as
Which of the above underlined parts of the sentence is not appropriate?
Select the pair that best expresses a relationship similar to that expressed in the
pair:
water: pipe::
Q. No. 6 – 10 Carry Two Marks Each
Q.
Velocity of an object fired directly in upward direction is given by V = 80 − 32 t,
where t (time) is in seconds. When will the velocity be between 32 m/sec and 64
m/sec?
In a factory, two machines M1 and M2 manufacture 60% and 40% of the autocomponents respectively. Out of the total production, 2% of M1 and 3% of M2 are found to be defective. If a randomly drawn autocomponent from the combined lot is found defective, what is the probability that it was manufactured
by M2?
Following table gives data on tourists from different countries visiting India in the
year 2011.
Which two countries contributed to the one third of the total number of tourists who visited India in
2011?
If |−2X + 9| = 3 then the possible value of |−X| − X2 would be:
All professors are researchers
Some scientists are professors
Which of the given conclusions is logically valid and is inferred from the above
arguments:
Q. 11 – Q. 15 carry one mark each
Q.
N(CH3)3 and N(SiH3)3 are congeners, but around N-atom the former has pyramidal geometry
whereas the latter is nearly planar. The bonding responsible for planarity of N(SiH3)3 is
The type of electronic transition responsible for the yellow colour of K2CrO4 is
The given equation
where H, T and Cp are the enthalpy, temperature and heat capacity at constant pressure,
respectively, is called
Q. 14 - Q. 15 are questions with numerical answer.
Q.
The number of 2-center-2-electron bonds in anhydrous AlCl3 is ___________
When dissolved in water, the number of H+ ions released from a molecule of H3BO3 is _________
Q. 16 - Q. 25 carry two marks each.
Q.
In NaCl crystal, the arrangement and coordination number of the ions are
The solubility product In a 0.02 M solution of Ca(NO3)2, the solubility of Ca3(PO4)2 (in units of M) is
Identify the CORRECT product in the following reaction
The major product obtained in the following reaction is
Q. 20- Q. 21 are questions with numerical answer.
Q.
Iodine forms an anionic species Q in aqueous solution of iodide(I-). The number of lone pair(s) of
electrons on the central atom of Q is __________
(Important : you should answer only the numeric value)
The rate of a chemical reaction is tripled when the temperature of the reaction is increased from 298
K to 308 K. The activation energy (in kcal mol-1 K-1, up to one decimal place) for the reaction
is(Given R = 1.987cal mol-1 K-1) _____________
Common Data for Questions 22 and 23:
Consider the following SN2 reaction of optically pure 1-chloro-3-ethylcyclopentane (X).
Q.
The structure of Y in the above reaction is
Consider the following SN2 reaction of optically pure 1-chloro-3-ethylcyclopentane (X).
Q.
The absolute configuration of1-chloro-3-ethylcyclopentane (X) shown above is
Statement for Linked Answer Questions 24 and 25:
The molar conductance at infinite dilution of sodium acetate, sodium sulfate and sulfuric acid solutions are 91.0 ×10-4, 259.8×10-4 and 859.3×10-4S m2 mol-1, respectively.
Q.
The molar conductance at infinite dilution (in S m2 mol-1)of acetic acid is
The molar conductance at infinite dilution of sodium acetate, sodium sulfate and sulfuric acid solutions are 91.0 ×10-4, 259.8×10-4 and 859.3×10-4S m2 mol-1, respectively.
Q.
If the molar conductance of an acetic acid solution is15.2 ×10-4Sm2 mol-1,then the percentage (%)
dissociation of acetic acid in the solution will be
Q. 1 – Q. 10 carry one mark each.
Q.
Which one of the following statements is TRUE when a cell is kept in a hypotonic solution?
Which one of the following amino acids has a higher propensity for cis peptide bond
formation?
The length of an α-helix composed of 36 amino acid residues is
The order n for a given substrate concentration in an enzyme catalyzed reaction following Michaelis-Menten kinetics, is
Which one of the following amino acid residues is specifically recognised by chymotrypsin
during peptide bond cleavage?

















