Case Based Questions Test: Acids, Bases & Salts - 1 - Class 9 MCQ
15 Questions MCQ Test Advance Learner Course: Science Class 9 - Case Based Questions Test: Acids, Bases & Salts - 1
Read the following and answer any questions:
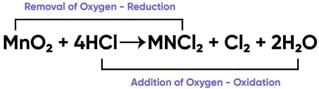
The reaction between MnO2 with HCl is depicted in the following diagram. It was observed that a gas with bleaching abilities was released.
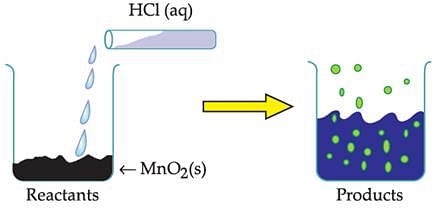
The chemical reaction between MnO2 and HCl is an example of:

Identify the correct statement from the following:
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
What will happen if we take dry HCl gas instead of aqueous solution of HCl?
Read the following and answer any questions:
Frothing in Yamuna:
The primary reason behind the formation of the toxic foam is high phosphate content in the wastewater because of detergents used in dyeing industries, dhobi ghat Yamuna’s pollution level is so bad that parts of it have been labelled ‘dead’ as there is no oxygen in it for aquatic life to survive.

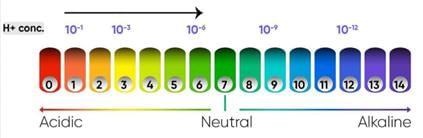
Predict the pH value of the water of river Yamuna if the reason for froth is high content of detergents dissolved in it.
The table provides the pH value of four solutions P, Q, R and S
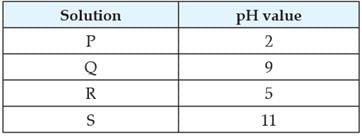
Which of the following correctly represents the solutions in increasing order of their hydronium ion concentration?
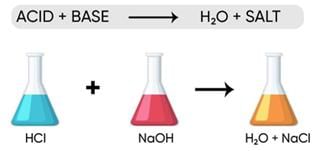
If a sample of water containing detergents is provided to you, which of the following methods will you adopt to neutralize it?
Study the given table and answer the following questions. It shows the pH value of the plaque (which collects around teeth) surrounding the teeth of a child over 3 hrs.
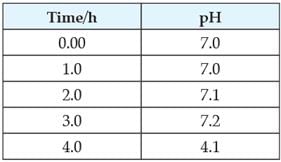
The three constituents of plaque are
State the time during the day when the condition is most favourable for the process of tooth decay.
Suhana takes three beakers A, B and C filled with aqueous solutions of glucose, alcohol and hydrochloric acid respectively as shown in the following figure:
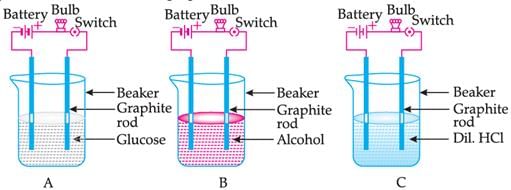
Which of the following statements is correct in terms of glowing of the bulb when the switch is ON?
Which of the following are present in a dilute aqueous solution of hydrochloric acid?
Study the given experimental set-up and answer the following questions.
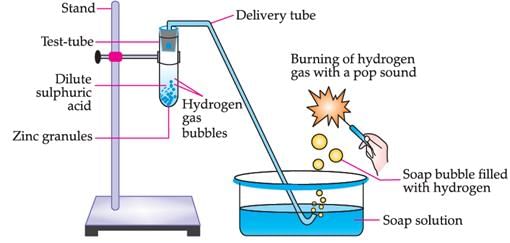
The above experimental set up shows reaction between metal and
Write the products formed in the above process:
Zn (s) + H 2 SO 4 (g) →
A metal is treated with dilute sulphuric acid. The gas evolved is collected by the method shown in the figure:
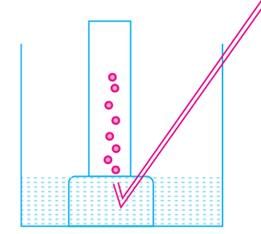
Name the gas evolved:
What nature of hydrogen is used as a fuel in rocket:
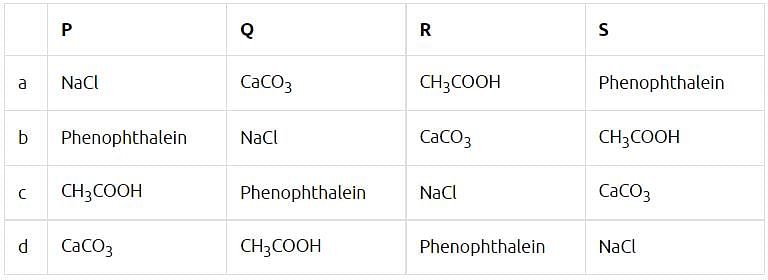
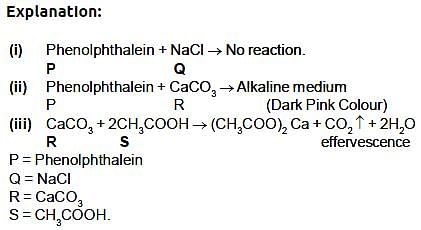
P, Q, R are different colourless solids, while S is a colourless solution. They are (in random order) Sodium chloride (NaCl), Calcium Carbonate (CaCO3), Acetic acid (CH3COOH) and Phenolphthalein indicator. Small amount of the above substances were added in pairs (e.g. P with Q; Q with R etc.) to a small amount of water in a test tube. They give the following results as shown in the observation table. Observation Table:
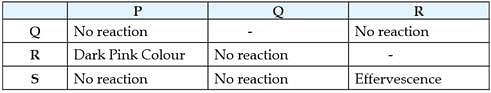
Which of the following reaction is incorrect:
|
11 videos|50 docs|17 tests
|
|
11 videos|50 docs|17 tests
|