Test: Structure Of The Atom- 2 - Class 9 MCQ
10 Questions MCQ Test Science Class 9 - Test: Structure Of The Atom- 2
In the Thomson’s model of atoms, which of the following statements are correct?
(i) The mass of the atoms is assumed to be uniformly distributed over the atom.
(ii) The positive charge is uniformly distributed over the space.
(iii) The electrons are uniformly distributed in the positively charged sphere.
(iv) The electrons attract each other to stabilise the atom.
(i) The mass of the atoms is assumed to be uniformly distributed over the atom.
(ii) The positive charge is uniformly distributed over the space.
(iii) The electrons are uniformly distributed in the positively charged sphere.
(iv) The electrons attract each other to stabilise the atom.
Which of the following statements about Rutherford’s model of atoms are correct?
(i) Considered the nucleus as positively charged.
(ii) Established that the a-particles are four times as heavy as a hydrogen atom.
(iii) Can be compared to solar system.
(iv) Was in agreement with Thomson’s model.
(i) Considered the nucleus as positively charged.
(ii) Established that the a-particles are four times as heavy as a hydrogen atom.
(iii) Can be compared to solar system.
(iv) Was in agreement with Thomson’s model.
An atom with 3 protons and 4 neutrons will have a valency of
Which of the following statement is always correct?
Rutherford’s a-scattering experiment led to the conclusion that
Which of the following in figures given below do not represent Bohr's model of an atom incorrectly ?
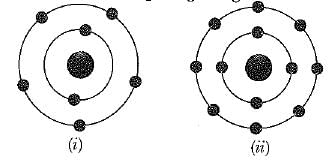
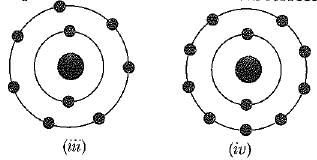
If K, L, M, N, shells of an atom are full. The total number of electrons in that atom are:
Atomic models have been improved over the years. Arrange the following atomic models in the order of their chronological order.
(i) Rutherford’s atomic model
(ii) Thomson’s atomic model
(iii) Bohr’s atomic model
Na+ has 12 neutrons and 10 electrons. Which of the following statement is correct?
|
88 videos|369 docs|67 tests
|



















