NTSE Level Test: Periodic Classification of Elements - Grade 9 MCQ
20 Questions MCQ Test Physical Science for High School - NTSE Level Test: Periodic Classification of Elements
Which of the following elements would lose an electron easily ?
Which of the following is non-metal and to which group does it belong?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which one of the following depict the correct representation of atomic radius (r) of an atom ?
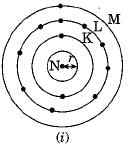
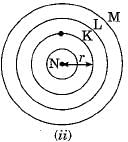
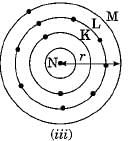
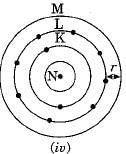




Silicon (14) belongs to class of
According to Mendeleev’s Periodic Law, the elements were arranged in the periodic table in the order of
Chlorine (17) belongs to which group and period
Which of the following statement(s) about Modern Periodic Table are incorrect ?
(i) The elements in the Modem Periodic Table are arranged on the basis of their decreasing atomic number.
(ii) The elements in the Modem Periodic Table are arranged on the basis of their increasing atomic masses.
(iii) Isotopes are placed in adjoining group(s) in the Periodic Table.
(iv) The elements in the Modern Periodic Table are arranged on the basis of their increasing atomic number.
Which of the following is most reactive
The concept of triads in the elements was given by
Which of the following is most reactive
Upto which element, the Law of Octaves was found to be applicable ?
Which of the following set of elements is written in order of their decreasing metallic character ?
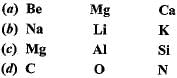
The elements A, B, C, D and E have atomic number 9, 11, 17, 12 and 13 respectively. Which pair of elements belong to the same group ?
Which one of the following does not increase while moving down the group of the periodic table ?
Which of the following statements is not a correct statement about the trends when going from left to right across the periods of periodic table ?
The element with atomic number 14 is hard and forms acidic oxide and a covalent halide. To which of the following categories does the element belong?
Element X forms a chloride with formula XCl2 which is a solid with a high melting point. X would most likely be in the same group of the Periodic Table as
Which of the following elements will form an acidic oxide ?
In Mendeleev’s Periodic Table, gaps were left for the elements to be discovered later. Which of the following elements found a place in the periodic table later ?
Which of the following elements does not lose an electron easily ?
|
79 videos|263 docs|65 tests
|
|
79 videos|263 docs|65 tests
|

















