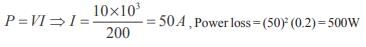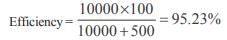WBJEE Phy & Chem Sample Paper - JEE MCQ
30 Questions MCQ Test WBJEE Sample Papers, Section Wise & Full Mock Tests 2025 - WBJEE Phy & Chem Sample Paper
One Kg of copper is drawn into a wire of 1mm diameter and a wire of 2 mm diameter. The resistance of the two wires will be in the ratio
An electrical cable having a resistance of 0.2 : delivers 10kw at 200V D.C. to a factory. What is the efficiency of transmission?
A wire of resistance 5Ω is drawn out so that its new length is 3 times its original length.What is the reistance of the new wire?
Two identical cells each of emf E and internal resistance r are connected in parallel with an external resistance R. To get maximum power developed across R, the value of R is
To write the decimal number 37 in binary, how many binary digits are required?
A junction diode has a resistance of 25Ω when forward biased and 2500Ω when reverse biased. The current in the diode, for the arrangement shown will be
If the electron in a hydrogen atom jumps from an orbit with level n1 = 2 to an orbit with level n2 = 1 the emitted radiation has a wavelength given by
What is the particle x in the following nuclear reaction:
An alternating current of rms value 10 A is passed through a 12Ω resistor. The maximum potential difference across the resistor is
Which of the following relation represent Biot-Savart’s law?
are two vectors given by
The magnitude of the component of
is
The acceleration ‘a’ (in ms–2) of a body, starting from rest varies with time t (in s) following the equation a = 3t + 4 The velocity of the body at time t = 2s will be
Figure below shows the distance-time graph of the motion of a car. If follows from the graph that the car is
Two particles have masses m & 4m and their kinetic energies are in the ratio 2: 1. What is the ratio of their linear momenta ?
The force F acting on a particle moving in a straight line is shown below. What is the work done by the force on the particle in the 1st meter of the trajectory ?
If the kinetic energy of a body changes by 20% then its momentum would change by –
A bullet is fired with a velocity u making an angle of 60° with the horizontal plane. The horizontal component o the velocity of the bullet when it reaches the maximum height is
A particle is projected at 60° to the horizontal with a kinetic energy K. The kinetic energy at the highest point is
The poisson’s ratio of a material is 0.5. If a force is applied to a wire of this material, there is a decrease in the cross-sectional area by 4%. The percentage increase in the length is :
Two spheres of equal masses but radii r1 and r2 are allowed to fall in a liquid of infinite column. The ratio of their terminal velocities is
Two massless springs of force constants K1 and K2 are joined end to end. The resultant force constant K of the system is
A spring of force constant k is cut into two equal halves. The force constant of each half is
Two rods of equal length and diameter have thermal conductivities 3 and 4 units respectively. If they are joined in series, the thermal conductivity of the combination would be
19 g of water at 30° C and 5 g of ice at – 20° C are mixed together in a calorimeter. What is the final temperature of the mixture? Given specific heat of ice = 0.5 cal g–1(°C)–1 and latent heat of fusion of ice = 80 cal g–1
. It is difficult to cook rice in an open vessel by boiling it at high altitudes because of
The height of a waterfall is 50 m. If g = 9.8 ms–2 the difference between the temperature at the top and the bottom of the waterfall is:
The distance between an object and a divergent lens is m times the focal length of the lens. The linear magnification produced by the lens is
A 2.0 cm object is placed 15 cm in front of a concave mirror of focal length 10 cm. What is the size and nature of the image?
A beam of monochromatic blue light of wavelength 4200 Å in air travels in water of refractive index 4/3. Its wavelength in water will be:
|
3 videos|10 docs|54 tests
|
|
3 videos|10 docs|54 tests
|