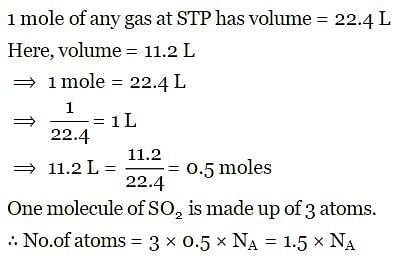BITSAT Chemistry Test - 3 - JEE MCQ
30 Questions MCQ Test BITSAT Mock Tests Series & Past Year Papers 2025 - BITSAT Chemistry Test - 3
In which of the following reactions, aldehydes and ketones are distinguished?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
which of the following hybridisation occurs in the formation of methane molecule?
Which of the following explains the sequence of filling electrons in different subshells?
Which of the following statements in relation to the hydrogen atom is correct?
The number of atoms contained in 11.2 L of SO₂ at S.T.P. are
The units nanometer, fermi, angstrom and attometre arranged in decreasing order
A carboxylic acid is converted into its anhydride using
Which chloride should exhibit the most covalent type of bond?
A vessel at equilibrium, contains SO₃, SO₂ and O₂. Now some helium gas is added, so that total pressure increases while temperature and volume remain constant. According to Le-Chatelier's principle, the dissociation of SO₃
The correct order of electron affinity among the following is
Which of the following is a common donor atom in ligands ?
Which of the following characteristics of the transition metals is associated with their catalytic activity?
The mass of carbon anode consumed (giving only carbon dioxide) in the production of 270 kg of aluminium from bauxite by Hall process is (Atomic weight of AI = 27)
Which one of the following alkenes will react faster with H₂ catalytic hydrogenation conditions ?
Which of the following compound is used to prevent the deposition of oxides of lead on spark plug, combustion chamber and exhaust pipe?
In which of the following acid-base titration pH is greater than8 at the equivalence point ?
The total number of acyclic isomers including stereoisomers of C₄H₇Cl are
If for a given substance, melting point is TB and freezing point is TA then which of following graph gives correct variation of entropy change vs temperature?
The element with atomic no. 36 belongs to which of the following block in the Periodic Table?
|
2 videos|17 docs|85 tests
|
|
2 videos|17 docs|85 tests
|