VITEEE PCBE Mock Test - 15 - JEE MCQ
30 Questions MCQ Test VITEEE: Subject Wise and Full Length MOCK Tests - VITEEE PCBE Mock Test - 15
Numerous vascular bundles occur scattered in the ground tissue of
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
These questions are based on the following information. P, Q, R, S and T sit around a table. P sits two seats to the left of R and Q sits two seats to the right of R.
Q. If S is not sitting next to Q, who is sitting between Q and S?
These questions are based on the following information. P, Q, R, S and T sit around a table. P sits two seats to the left of R and Q sits two seats to the right of R.
Q. If a new person U joins the group such that the initial conditions for the seating arrangement should be observed and also a new condition that U does not sit next to R be satisfied, then which of the following statements is true?
These questions are based on the following information. P, Q, R, S and T sit around a table. P sits two seats to the left of R and Q sits two seats to the right of R.
Q. If a new person U joins the group such that the initial conditions for the seating arrangement should be observed and also a new condition that U does not sit next to P, S or T be satisfied, then who will be the neighbours of P (one on either side)?
Directions: Study the following graph and answer the question that follows.
POWER PRODUCTION OF A COMPANY (IN MILLION WATTS)
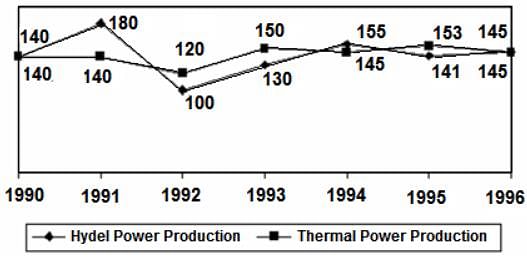
Q. What is the difference between the peak hydel power production and the least Hydel Power Production ?
Directions: Study the following graph and answer the question that follows.
POWER PRODUCTION OF A COMPANY (IN MILLION WATTS)
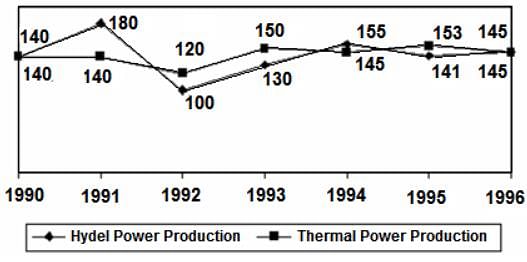
Q. In which year is the total power production (thermal + hydel) the greatest?
Directions: Study the following graph and answer the question that follows.
POWER PRODUCTION OF A COMPANY (IN MILLION WATTS)
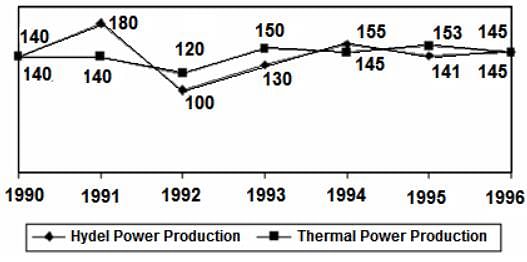
Q. What is the average thermal power production for the period 1990-1996?
Directions: Study the following graph carefully and answer the question given below.
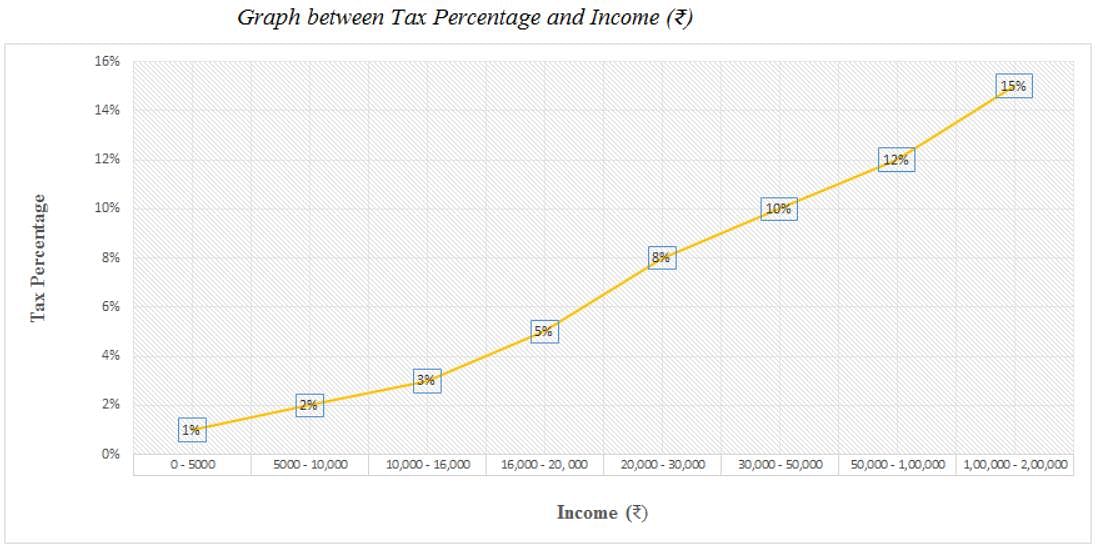
Q. If the population of a city is 35,000 and the average income of a person in that city is Rs. 25,000 per annum, then what is the total amount of tax paid by all the persons of that city?
In some code, letters a, b, c, d and e represent numbers 2, 4, 5, 6 and 10. We just do not know which letter represents which number. Consider the following relationships:
I. a + c = e,
II. b – d = d and
III. e + a = b
Which of the following options are true?
The sum of the first 100 natural numbers, 1 to 100 is divisible by:
In a four-digit number, the sum of the first 2 digits is equal to that of the last 2 digits. The sum of the first and last digits is equal to the third digit. Finally, the sum of the second and fourth digits is twice the sum of the other 2 digits. What is the third digit of the number?
|
1 videos|2 docs|73 tests
|
|
1 videos|2 docs|73 tests
|


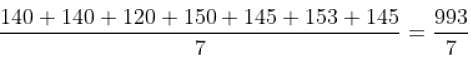 = 141.85 million watts
= 141.85 million watts














