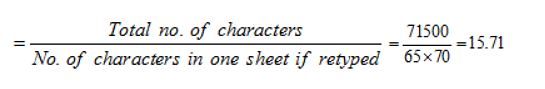VITEEE PCBE Mock Test - 3 - JEE MCQ
30 Questions MCQ Test VITEEE: Subject Wise and Full Length MOCK Tests - VITEEE PCBE Mock Test - 3
In which of the following animals, blood does not transport oxygen
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which type of blood vessel has thick walls in order to withstand high pressure?
The free floating organisms, of an open sea and shore, are collectively called
Out of two alleles of the same gene, one finds morphological expression. The phenomenon is
How many types of genotypes are formed in F₂-progeny obtained from self-pollination of a dihybrid F₁ ?
A pregnant woman underwent amniocentesis. An extra barr body is present in the embryo. Syndrome likely to occur in child is
A disease transferred from mother to child through placenta is
Directions: Study the following information and answer the question that follows.
The table below shows the percentage profit or loss of five different commodities based on the sum of cost price and shipping cost.
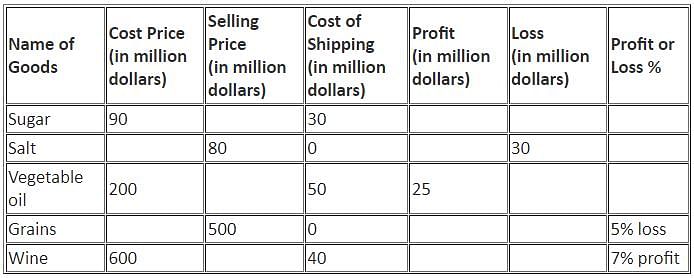
Q. What is the Percentage of the Selling price of vegatable oil and cost price of salt ?
Directions: Study the following information and answer the question that follows.
The table below shows the percentage profit or loss of five different commodities based on the sum of cost price and shipping cost.
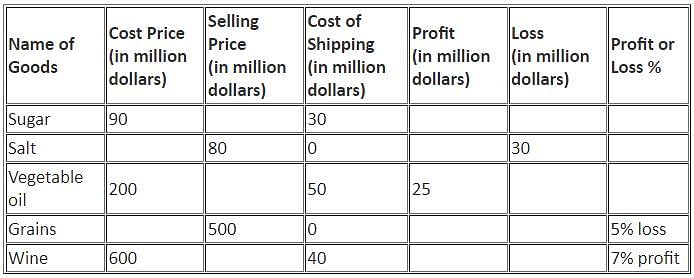
Q. What is the difference between the selling price of Wine and that of Vegetable oil?
Directions: Study the following information and answer the question that follows.
The table below shows the percentage profit or loss of five different commodities based on the sum of cost price and shipping cost.
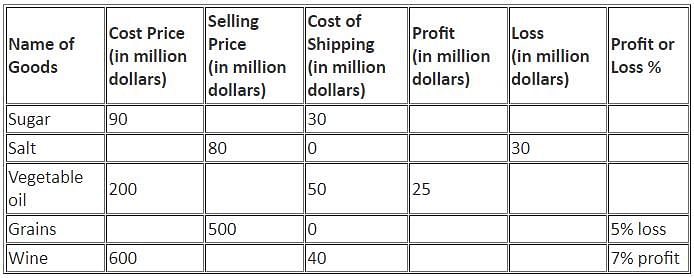
Q. What is the ratio of the loss on Grains to that on Salt?
Directions: Study the given information and answer the following question.
The table given below shows the estimated cost (in Rs. lakh) of a project of laying a railway line between two places, the project began in 1988 and was completed in 1991.
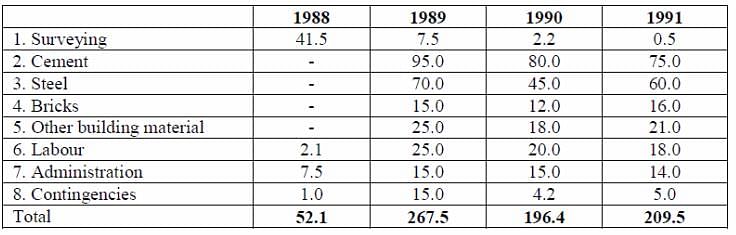
Q. How many times approximately was the total cost of materials to the total labour cost for all the years?
Anil buys 12 toys and labels each with the same selling price. He sells 8 toys initially at 20% discount on the labeled price. Then he sells the remaining 4 toys at an additional 25% discount on the discounted price. Thus, he gets a total of Rs 2112, and makes a 10% profit. With no discounts, his percentage of profit would have been
A person who has a certain amount with him goes to market. He can buy 50 oranges or 40 mangoes. He retains 10% of the amount for taxi fares and buys 20 mangoes and of the balance, he purchases oranges. Number of oranges he can purchase is:
A report consists of 20 sheets each of 55 lines and each such line consists of 65 characters. This report is reduced onto sheets each of 65 lines such that each line consists of 70 characters. The percentage reduction in number of sheets is closest to:
Directions: Study the following graph carefully and answer the questions given below:
Production (in Lakhs) of Widgets & Percentage defect over the years in five factories A, B, C, D, E.
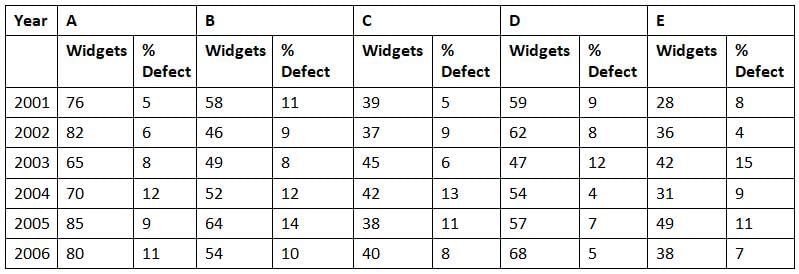
Q. The average number of widgets that were produced in the year 2002 formed what percent of the widgets that were produced in the year 2006?
Directions: Study the following graph carefully and answer the questions given below:
Production (in Lakhs) of Widgets & Percentage defect over the years in five factories A, B, C, D, E.
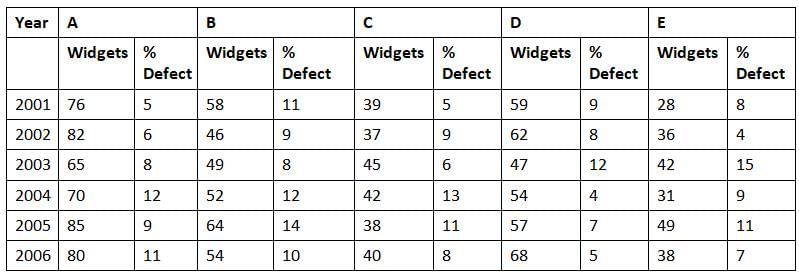
Q. What was the difference in the number of widgets of factory C which were free from any defect in the years 2004 and 2006?
Directions: Study the following table carefully and answer the questions given below.
Number of candidates appeared (App.) and percentage of candidates qualified (Qual.) under different disciplines over the years.

Q. Approximately, what was the percentage increase in the number of candidates who appeared under the German stream from 2013 to 2014(approx)?
|
1 videos|2 docs|73 tests
|
|
1 videos|2 docs|73 tests
|