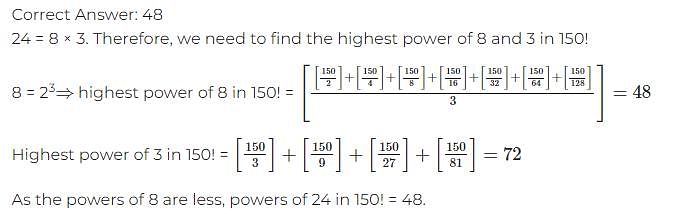VITEEE PCBE Mock Test - 6 - JEE MCQ
30 Questions MCQ Test VITEEE: Subject Wise and Full Length MOCK Tests - VITEEE PCBE Mock Test - 6
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Directions: The question is based on the following pie chart.
Project implementation cost of ABC Ltd. (Rs. Crores)
Total project cost = Rs. 252 Crores
If the plant cost increases by 5% due to increase in construction cost, what will be approximate angle of the plant sector in the pie chart?
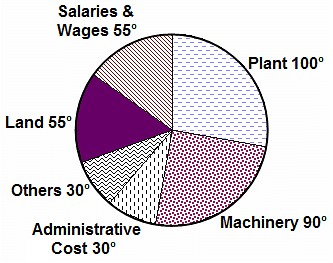
Directions: The question is based on the following pie chart.
Project implementation cost of ABC Ltd. (Rs. Crores)
Total project cost = Rs. 252 Crores
If there is an escalation of 10% in Salaries& Wages and a reduction of 10% in land, what will be the new angle of salaries and wages?
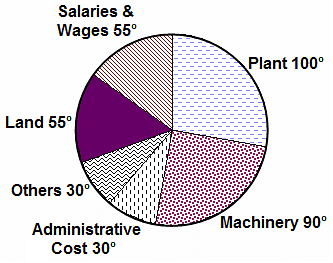
Directions: The question is based on the following pie chart.
Project implementation cost of ABC Ltd. (Rs. Crores)
Total project cost = Rs. 252 Crores
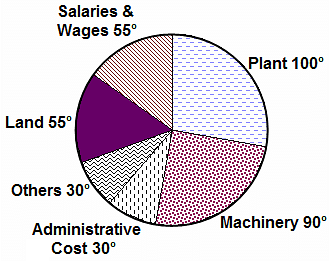
If 5% of administrative costs are reduced and 10% of the remaining administrative costs are transferred to Others category, what is the angle of administrative costs now?
Directions: The following diagrams show the cost price and selling price (in Rs.) of different fruits sold by a fruit vendor.
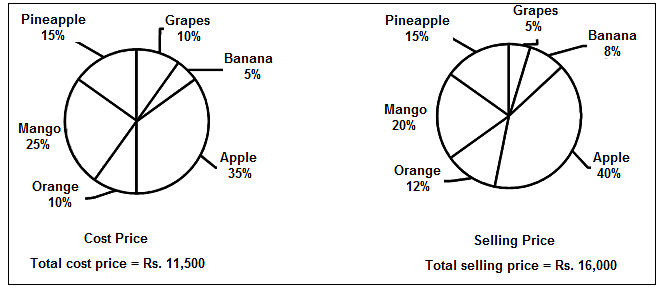
How much percentage profit can he earn from the apples?
Look at this series: 3, 4, 7, 8, 11, 12,...so on. What number should come next?
Look at this series: 8, 22, 8, 28, 8,...so on. What number should come next?
Look at this series: 1, 3, 6, 10, 15...so on. What number should come next?
After the division of a number successively by 3, 4 and 7, the remainders obtained are 2, 1 and 4 respectively. What will be the remainder if 84 divides the same number?
Teacher said that there were 100 students in his class, 24 of whom were boys and 32 were girls. Which base system did the teacher use in this statement?
|
1 videos|2 docs|73 tests
|
|
1 videos|2 docs|73 tests
|


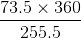 = 103.6° (Approx)
= 103.6° (Approx)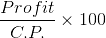 =
=