VITEEE PCBE Mock Test - 7 - JEE MCQ
30 Questions MCQ Test VITEEE: Subject Wise and Full Length MOCK Tests - VITEEE PCBE Mock Test - 7
Cork is an excellent material for making bottle stopper because it is
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
A dog sees a cat. It estimates that the cat is 25 leaps away. The cat sees the dog and starts running with the dog in hot pursuit. If in every minute, the dog makes 5 leaps and the cat makes 6 leaps and one leap of the dog is equal to 2 leaps of the cat. Find the time in which the cat is caught by the dog (assume an open field with no trees).
Two cars started simultaneously towards each other and met each other 3 h 20 min later. How much time will it take the slower car to cover the whole distance if the first arrived at the place of departure of the second 5 hours later than the second arrived at the point of departure of the first?
Charlie and Alan run a race between points A and B, 5 km apart. Charlie starts at 9 a.m. from A at a speed of 5 km/hr, reaches B, and returns to A at the same speed. Alan starts at 9:45 a.m. from A at a speed of 10 km/hr, reaches B and comes back to A at the same speed. At what time do Charlie and Alan first meet each other?
Two rabbits start simultaneously from two rabbit holes towards each other. The first rabbit covers 8% of the distance between the two rabbit holes in 3 hours, The second rabbit covered 7 / 120 of the distance in 2 hours 30 minutes. Find the speed (feet / h) of the second rabbit if the first rabbit travelled 800 feet to the meeting points.
Directions: Study the following graph carefully and answer the question given below.
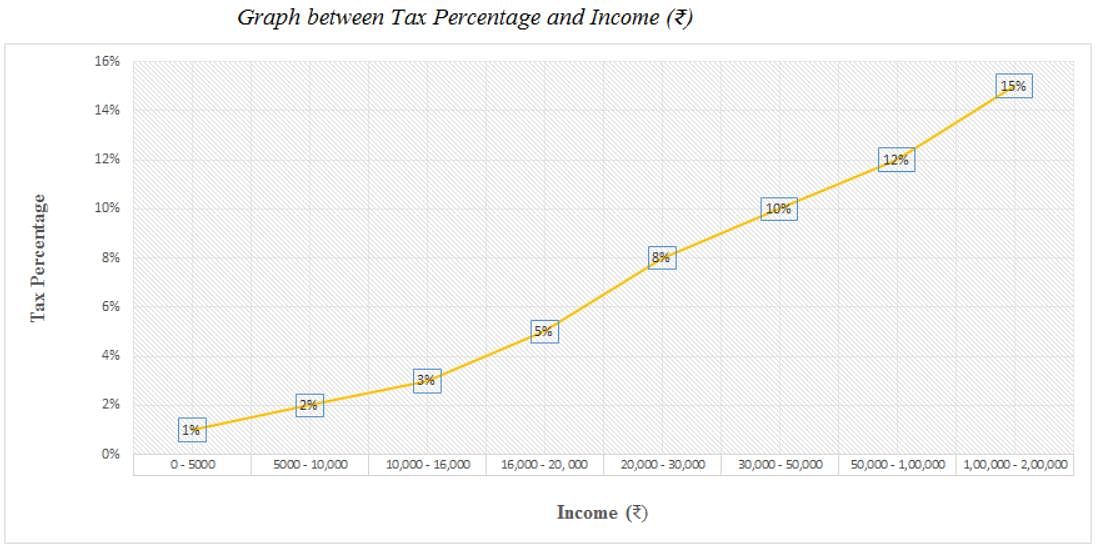
Q. What is the total amount of tax on an income of Rs. 9000 and an income of Rs. 2000?
Directions: Study the following graph carefully and answer the question given below.
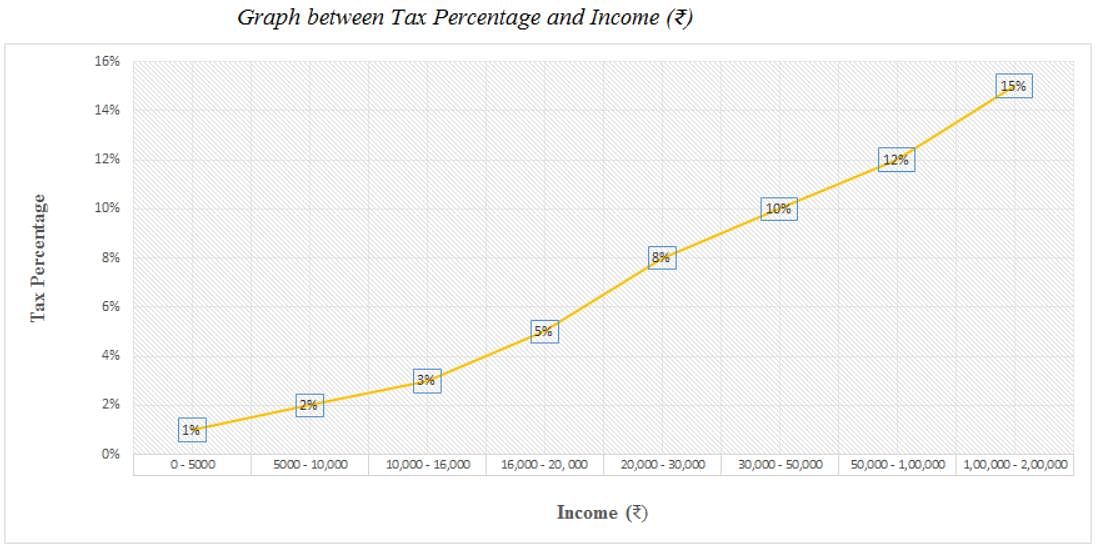
Q. What is the ratio of the tax on an income of Rs. 15,550 to that on an income of Rs. 16,550?
Directions: Refer to the graph and answer the following question.
The line graph below gives information about the number of applications received by different colleges A, B and C for admission to their MBA programme from 2012 to 2016.
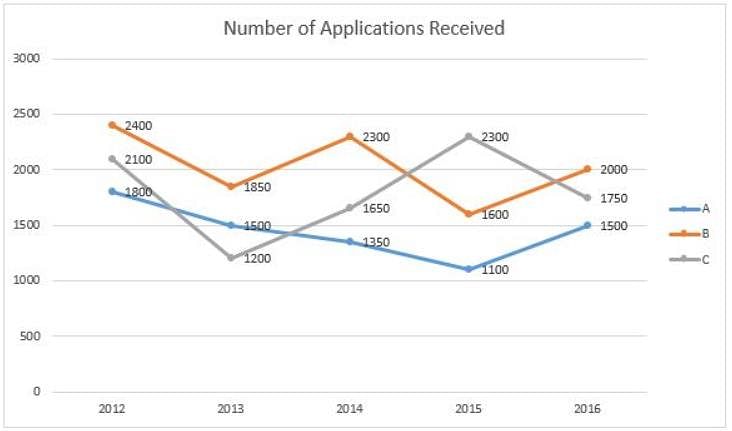
Q. What is the ratio of the number of applications received by colleges A, B and C in 2013 to that in 2016?
Directions: Refer to the graph and answer the following question.
The line graph below gives information about the number of applications received by different colleges A, B and C for admission to their MBA programme from 2012 to 2016.
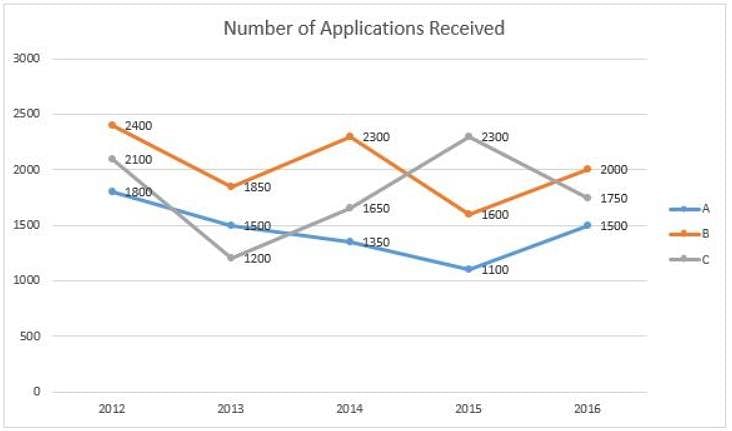
Q. What is the difference between the number of applications received by college C during 2013-2016 and that received by college A during 2012-2015?
Instead of a metre scale, a cloth merchant uses a faulty 120 cm scale while buying, but uses a faulty 80 cm scale while selling the same cloth. If he offers a discount of 20%, what is his overall profit percentage?
Sailesh is working as a sales executive with a reputed FMCG Company in Hyderabad. As per the Company’s policy, Sailesh gets a commission of 6% on all sales upto Rs. 1,00,000 and 5% on all sales in excess of this amount. If Sailesh remits Rs. 2,65,000 to the FMCG company after deducting his commission, his total sales were worth:
|
1 videos|2 docs|73 tests
|
|
1 videos|2 docs|73 tests
|

















