VITEEE PCBE Mock Test - 8 - JEE MCQ
30 Questions MCQ Test VITEEE: Subject Wise and Full Length MOCK Tests - VITEEE PCBE Mock Test - 8
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Directions: Study the following graph carefully and answer the question given below.
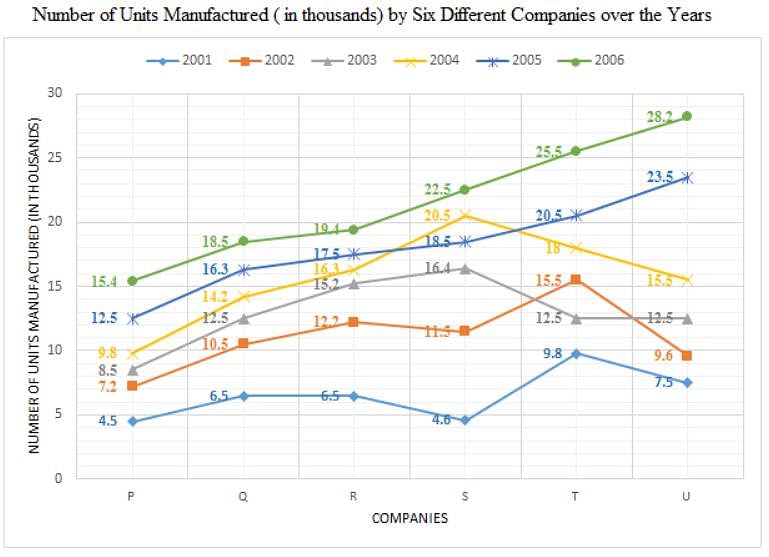
Q. The total number of units manufactured by companies R, S and U in the year 2006 is what percentage of the total number of units manufactured by Company P in all the years together?
Directions: Study the following graph carefully and answer the question given below.

Q. What is the average number of units sold by Company S in the years 2001, 2002, 2003, 2004 and 2005?
Directions: Study the following graph carefully and answer the question given below.

Q. What is the respective ratio of total number of units manufactured by companies Q and R together in the year 2001 to those manufactured by companies S and T together in the year 2003?
Directions: Study the following graph carefully and answer the question given below.
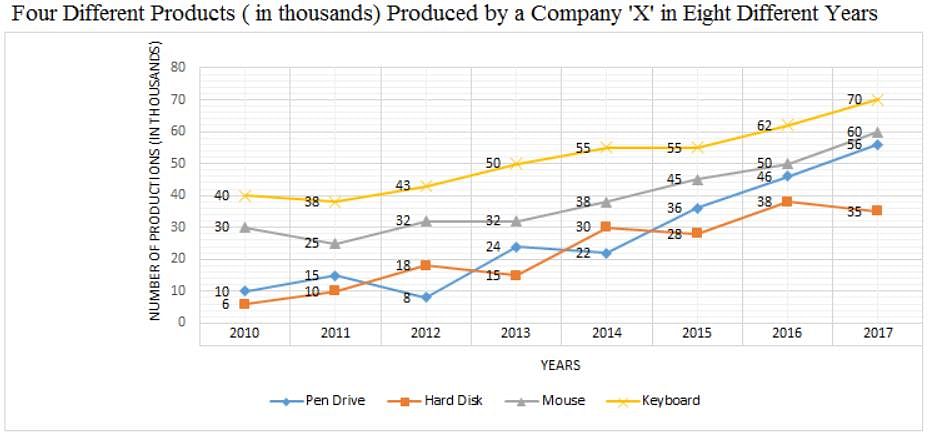
Q. What is the average number of hard disks produced by Company 'X' over all the years together?
Rohan purchased some pens, pencils and erasers for his young brothers and sisters for the ensuing examinations. He had to buy atleast 11 pieces of each item in a manner that the number of pens purchased is more than the number of pencils, which is more than the number of erasers. He purchased a total of 38 pieces. If the number of pencils cannot be equally divided among his 4 brothers and sisters, how many pens did he purchase?
A nursery has 363, 429 and 693 plants respectively of 3 distinct varieties. It is desired to place these plants in straight rows of plants of 1 variety only so that the number of rows required is the minimum. What is the size of each row and how many rows would be required?
Shyam visited Ram during his brief vacation. In the mornings they both would go for yoga. In the evenings they would play tennis. To have more fun, they indulge only in one activity per day, i.e. either they went for yoga or played tennis each day. There were days when they were lazy and stayed home all day long. There were 24 mornings when they did nothing, 14 evenings when they stayed at home, and a total of 22 days when they did yoga or played tennis. For how many days Shyam stayed with Ram?
How many even integers n, where 100 ≤ n ≤ 200, are divisible neither by seven nor by nine?
There are 3 clubs A, B & C in a town with 40, 50 & 60 members respectively. While 10 people are members of all 3 clubs, 70 are members in only one club. How many belong to exactly two clubs?
|
1 videos|2 docs|73 tests
|
|
1 videos|2 docs|73 tests
|

















