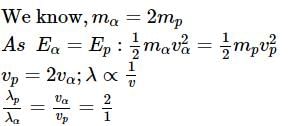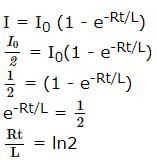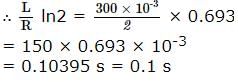AIIMS Full Mock Test - 10 - NEET MCQ
30 Questions MCQ Test AIIMS Mock Tests & Previous Year Question Papers - AIIMS Full Mock Test - 10
A 40Ω electric heater is connected to a 200 V, 50 Hz mains supply. The peak value of electric current flowing in the circuit is approximately
The threshold wavelength of certain metal is 2000 Å , its work function is
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
When a resistance of 2 ohm is connected across the terminals of a cell, the current is 0.5 amperes. When the resistance is increased to 5 ohm, the current is 0.25 amperes. The internal resistance of the cell is
A gun fires a bullet of mass 50 gm with a velocity of 30 m sec⁻1. Because of this gun is pushed back with a velocity of 1 m sec⁻1. The mass of the gun is
A parallel plate capacitor is filled by copper plate of thickness b. The new capacity will be
What will be the ratio of de Broglie wavelengths of proton and α-particle of same energy
A photoelectric cell is illuminated by a point source of light 1 m away. When the source is shifted to 2 m then
Gases begin to conduct electricity at low pressure because
The curve drawn between velocity and frequency of photon in vacuum will be a
The current passing through a coil of 5 H is decreasing at the rate of 2 A/s. The emf developed in the coil is
If the flux of magnetic induction through a coil of resistance R and having N turns changes from φ 1 to φ 2 , then the magnitude of the charge that passes through this coil is
A coil of inductances 300mH and resistance 2 Ω is connected to a source of voltage 2V. The current reaches half of its steady state value in
In an electromagnetic wave, the electric and magnetising fields are 100 Vm-1 and 0.265 Am-1. The maximum energy flow is
If the distance between the plates of a parallel plate condenser is halved and the dielectric is doubled, then its capacity increases by
The capacitance of a parallel plate capacitor is 10 μF when distance between its plates is 8 cm. If distance between the plates is reduced to 4 cm, its capacitance will be
Two masses of 1 gm and 4 gm are moving with equal kinetic energies. The ratio of the magnitudes of their linear momentum is
If the unit of force and length be each increased by four times, then the unit of energy is increased by
At the magnetic poles of the earth, a compass needle will be
The meniscus of a liquid contained in one of the limbs of a narrow U-tube is held in an electromagnet with the meniscus in line with the field. The liquid is seen to rise. This indicates that the liquid is
An electric kettle takes 4A current at 220 V . How much time will it take to boil 1 kg of water from temperature 20ºC ? The temperature of boiling water is 100ºC.
A uniform plank of Young's modulus Y, is moved over a smooth horizontal surface by a constant horizontal force F. The area of cross-section of plank is A. The compressive strain on the plank in the direction of the force is
A person sitting in an open car moving at constant velocity throws a ball vertically up into air. The ball falls
A mass m = 100 gms is attached at the end of a light spring which oscillates on a frictionless horizontal table with an amplitude equal to 0.16 metres and time period equal to 2 sec. Initially the mass is released from rest at t = 0 and displacement x = - 0.16 m. The expression for the displacement of the mass at any time (t) is
In the following question, a Statement of Assertion (A) is given followed by a corresponding Reason (R) just below it. Read the Statements carefully and mark the correct answer-
Assertion(A): When a massive projectile collides with a lighter stationary target then maximum speed of target is twice that of projectile.
Reason(R): It is explained by the momentum and energy conservation.
In the following question, a Statement of Assertion (A) is given followed by a corresponding Reason (R) just below it. Read the Statements carefully and mark the correct answer-
Assertion(A): A falling ball is attracted by the earth, because of which velocity of the ball goes on increasing in the downward direction. The ball also attracts the earth with same force but velocity developed in earth is negligible.
Reason(R): Momentum of ball and earth system is conserved.
In the following question, a Statement of Assertion (A) is given followed by a corresponding Reason (R) just below it. Read the Statements carefully and mark the correct answer-
Assertion(A): The maximum frequency of the photon produced by the union of a positron and an electron is 1.2 x 1020Hertz.
Reason(R): In electron-positron collision, both are annihilated and the total mass is converted into γ-photon(energy).
In the following question, a Statement of Assertion (A) is given followed by a corresponding Reason (R) just below it. Read the Statements carefully and mark the correct answer-
Assertion(A): For stable equilibrium, Force has to be zero and potential energy should be minimum.
Reason(R): For equilibrium, it is not necessary that the force is not zero.
Which of the following is the best example of an ideal black body
|
3 videos|44 docs|66 tests
|
|
3 videos|44 docs|66 tests
|