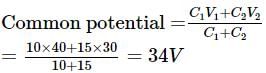AIIMS Full Mock Test - 11 - NEET MCQ
30 Questions MCQ Test AIIMS Mock Tests & Previous Year Question Papers - AIIMS Full Mock Test - 11
In the photo electric phenomenon, the number of photo electrons emitted is proportional to
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
What will be the ratio of de Broglie wavelengths of proton and α-particle of same energy
To obtain 3 μF capacity from three capacitors of 2 μF each, they will be arranged
A fuse wire of 5 A can withstand a maximum power of 1 W in the circuit. Resistance of the fuse wire is
The relation F = Ma, can not be deduced from Newton's second law if
The mass of a particle is 400 times than that of an electron and the charge is double. The particle is accelerated by 5 V. Initially the particle remained in rest, then its final kinetic energy will be
The energy of a photon of light with wavelength 5000Å is approximately 2.5eV. This way the energy of an X-ray photon with wavelength 1Å would be
If we consider electrons and photons of the same wavelength, then they will have the same
What is self inductance of a which produces 5V, when current in it changes from 3A to 2A in one millisecond ?
What is the value of inductance L for which the current is a maximum in series LCR circuit with C = 10 μF and ω = 1000 s − 1 ?
Which one of the following electromagnetic radiations have the smallest wavelength?
A quatity X is given by ε0 L ΔV /Δt , where ε0 is the permittivity of free space, L is a length, ΔV is potential difference and Δt is the time interval. The dimensional formula for X is the same as that of
A capacitor of capacity 10 μF is charged to 40V and a second capacitor of capacity 15 μF is charged to 30V. If they are connected in parallel the amount of charge that flows from the smaller capacitor to higher capacitor in μC is
If the K.E of a particle is doubled, then its momentum will
If the kinetic energy of a body is increased by 300%, its momentum will increased by
The rates of cooling of two different liquids put in exactly similar calorimeters and kept in identical surroundings are the same if
There are two bulbs A and B. Bulb A has power 300 W and operating at 220 V. Bulb B has power 500 W and operating at same voltage. Then ratio of resistance offered by A and B will be
A dip circle is so set that its needle moves freely in the magnetic meridian. In this position, the angle of dip is 40º. Now the dip circle is rotated so that the plane in which the needle moves makes an angle of 30º with the magnetic meridian. In this position, the needle will dip by an angle
Which of the following affets the elasticity of a substance?
A man is standing on a balance and his weight is measured. If he takes a step in the left side. then weight
A radioactive sample has half life of 1500 years. A sealed tube containing 1 g of a sample will be containing ........... of the sample after 3000 years. The missing figure is
A body is executing S.H.M. when its displacement from the mean position is 4 cm and 5 cm, the corresponding velocity of the body is 10 cm/sec and 8 cm/sec. Then the time period of the body is
In the following question, a Statement of Assertion (A) is given followed by a corresponding Reason (R) just below it. Read the Statements carefully and mark the correct answer-
Assertion(A): A parallel plate capacitor is connected across battery through a key. A dielectric slab of constant K is introduced between the plates. The energy stored becomes K times.
Reason(R): The surface density of charge on the plates remains constant or unchanged.
In the following question, a Statement of Assertion (A) is given followed by a corresponding Reason (R) just below it. Read the Statements carefully and mark the correct answer-
Assertion(A): Virtual images are always erect.
Reason (R): Virtual images are formed by diverging lenses only.
In the following question, a Statement of Assertion (A) is given followed by a corresponding Reason (R) just below it. Read the Statements carefully and mark the correct answer-
Assertion(A): For refraction in a plane surface can be used for the light moving from μ1 to μ2.
Reason(R): for light getting into a medium of index μ2 from μ1.
In the following question, a Statement of Assertion (A) is given followed by a corresponding Reason (R) just below it. Read the Statements carefully and mark the correct answer-
Assertion(A): An electron moving antiparallel to the field experiences maximum force.
Reason(R): F → = q v → x B → (symbols have their usual meaning).
|
3 videos|44 docs|66 tests
|
|
3 videos|44 docs|66 tests
|


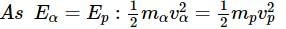
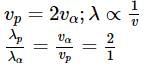
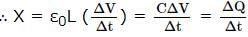 = current
= current