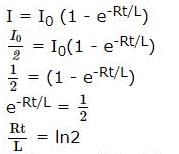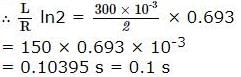AIIMS Full Mock Test 12 - NEET MCQ
30 Questions MCQ Test AIIMS Mock Tests & Previous Year Question Papers - AIIMS Full Mock Test 12
An electron remains undeflected when passing perpendicular to mutually perpendicular electric and magnetic fields. If the magnetic field is 8 Gauss and elecric field is 4000 V/m, the velocity of the electron is
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
A photon of energy 4 eV is incident on a metal surface whose work function is 2 eV. Minimum value of reverse potential to be applied for stopping the emission of electrons is
Two balls at same temperature collide. What is conserved ?
If momentum of a particle is doubled, then its de-Broglie's wavelength will
The potential difference across the terminals of a battery is 50V when 11A current is drawn and 60V when 1A current is drawn. The e.m.f. and the internal resistance of the battery are
Which of the following electromagnetic waves has minimum frequency ?
In Millikan oil drop experiment a drop of charge Q and radius r is kept constant between two plates of potential difference of 800 volt. Then charge on other drop of radius 2r which is kept constant with a potential difference of 3200 V is
A generator at a utility company produces 100 A of current at 4000 V. The voltage is stepped up to 240000 V by a transformer before it is sent on a high voltage transmission line. The current in transmission line is
In a pure inductive circuit with a.c. source, the current lags behind e.m.f. by
The number of turs of primary and secondary coils of a transformer are 5 and 10 respectively and mutual inductance of the transformer is 25 H. Now, number of turns in primary and secondary are made 10 and 5 respectuvely. Mutual inductance of transformer will be
A coil of inductances 300mH and resistance 2 Ω is connected to a source of voltage 2V. The current reaches half of its steady state value in
A charge q is located at the centre of a cube. The electric flux through any face is
When a dielectric material is kept in between the plates of a condenser, its capacity
A long spring is stretched by 2 cm, its potential energy is U. If the spring is stretched by 10 cm, the potential energy stored in it will be
If g is the acceleration due to gravity on the earth's surface, the gain in the potential energy of an object of mass m raised from the surface of earth to a height equal to the radius of the earth R, is
A 10 microfarad capacitor is charged to 500 V and then its plates are joined together through a resistance of 10 ohm. the heat produced in the resistance is
A frog can be levitated in magnetic field produced by a current in a vertical solenoid placed below the frog. This is possible because the body of the frog behaves as
The effective length of a magnet is 31.4 cm, and its pole strength is 0.5 A-m. If it is bent in the form of a semicircle,then its new magnetic moment will be
A wire of length L and radius a rigidly fixed at one end. On stretching the other end of the wire with a force F, the increase in it length is l. If another wire of same material but of length 2L and radius 2a is stretched with a force 2F, the increase in its length will be
In a simple harmonic motion displacement is half of its amplitude, what will be the ratio of K.E. and P.E.
In the following question, a Statement of Assertion (A) is given followed by a corresponding Reason (R) just below it. Read the Statements carefully and mark the correct answer-
Assertion(A): A particle of mass m collides with another stationary particle of mass M. If the particle m stops just after the collision, the coefficient of restitution of collisions is equal to 1.
Reason(R): Momentum of system just before and after the collision remains constant.
In the following question, a Statement of Assertion (A) is given followed by a corresponding Reason (R) just below it. Read the Statements carefully and mark the correct answer-
Assertion(A): Size of U235 is not essential for the phenomenon of nuclear fission.
Reason(R): Chain reaction takes place in nuclear fission.
In the following question, a Statement of Assertion (A) is given followed by a corresponding Reason (R) just below it. Read the Statements carefully and mark the correct answer-
Assertion(A): Magnetic induction inside a straight solenoid of finite length is μ₀ni.
Reason(R): 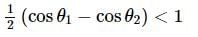
In the following question, a Statement of Assertion (A) is given followed by a corresponding Reason (R) just below it. Read the Statements carefully and mark the correct answer-
Assertion(A): When net force is perpendicular to direction of velocity, it just changes its direction and not magnitude.
Reason (R): This results in uniform circular motion.
|
3 videos|44 docs|66 tests
|
|
3 videos|44 docs|66 tests
|