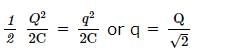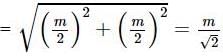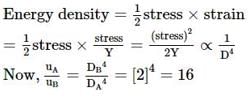AIIMS Full Mock Test 13 - NEET MCQ
30 Questions MCQ Test AIIMS Mock Tests & Previous Year Question Papers - AIIMS Full Mock Test 13
An alternating current is given by the equation i = i1 cos ωt + i2 sin ωt . The rms current is given by
The number of electrons emitted by a surface exposed to light is directly propotional to
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
4 eV is the energy of the incident photon and the work function in 2 eV. What is the stopping potential
A parallel plate capacitor is filled by copper plate of thickness b. The new capacity will be
An energy source will supply a constant current into the load, if its internal resistance is
A heavy steel ball of mass greater than 1Kg moving with a speed of 2ms-1 collides head on with a stationary ping pong ball of mass less than 0.1g. The collision is elastic. After the collision the ping pong ball moves approximately with a speed
When the speed of electrons increases, then the value of its specific charge
If a radiation of energy E falls normally on a perfectly reflecting surface, then the momentum transferred to the surface is
A 100 mH coil carries a current of 1 ampere. Energy stored in it is
In an ideal parallel LC circuit, the capacitor is charged by connecting it to a.c.d. source, which is then disconnected. The current in the circuit
The ratio of charge to potential of a capacitor is known as its
In an oscillating LC circuit the maximum charge on the capacitor is Q. The charge on the capacitor when the energy is stored equally between the electric and magnetic fields is
A paralel plate capacitor of capacity 100 μ F is charged by a battery of 50 volts. The battery ramins connected and if the plates of the capacitor are separated so that the distance between them become double the original distance, the additional energy given by the battery to the capacitor in joules is
Two masses of 1 gm and 4 gm are moving with equal kinetic energies. The ratio of the magnitudes of their linear momentum is
The temperature of a body falls from 50ºC to 40ºC in 10 minutes. If the temperature of the surroundings is 20ºC Then temperature of the body after another 10 minutes will be
The resistance of two bulbs of the same voltage are in the ratio 1:2. On connecting them in parallel the power consumption will be in the ratio
A magnet of magnetic moment M is cut into two equal parts. The two parts are placed perpendicular to each other so that their north poles touch each other. The resultant magnetic moment is
At the magnetic poles of the earth, a compass needle will be
Two wires of the same material and length, but diameters in the ratio 1 : 2 are stretched by the same force. The elastic potential energy stored per unit volume for the two wires when stretched, will be in the ratio of
A person sitting in an open car moving at constant velocity throws a ball vertically up into air. The ball falls
Radioactive sodium in the form of sodium chloride, is used to detect
A particle executes simple harmonic motion with an angular velocity and maximum acceleration of 3.5 rad/sec and 7.5 m/s2 respectively. Amplitude of the oscillation is
In the following question, a Statement of Assertion (A) is given followed by a corresponding Reason (R) just below it. Read the Statements carefully and mark the correct answer-
Assertion(A): The roaring of a lion is of low pitch while the buzzing of a mosquito is of high pitch.
Reason(R): Pitch is that characteristic of sound which enables us to distinguish between a shrill sound and a grave sound.
In the following question, a Statement of Assertion (A) is given followed by a corresponding Reason (R) just below it. Read the Statements carefully and mark the correct answer-
Assertion(A): A galvonometer is said to be sensitive if a small current through it produces a small deflection.
Reason(R): Current sensitivity = 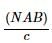 (symbols have their usual meaning).
(symbols have their usual meaning).
In the following question, a Statement of Assertion (A) is given followed by a corresponding Reason (R) just below it. Read the Statements carefully and mark the correct answer-
Assertion(A): The value of T such that v(t + T) = v(t) in a S.H.M. is 2π/ω.
Reason(R): A S.H.M. repeats at regular intervals called time period.
In the following question, a Statement of Assertion (A) is given followed by a corresponding Reason (R) just below it. Read the Statements carefully and mark the correct answer-
Assertion(A): If ℇ0 and μ0 respectively the electric permittivity and magnetic permeability of free space, and E and υ the corresponding quantities in a medium then refreactive index,
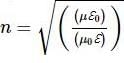
Reason(R): Speed of light in free space
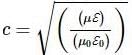
|
3 videos|44 docs|66 tests
|
|
3 videos|44 docs|66 tests
|