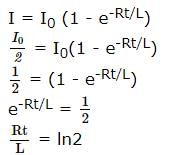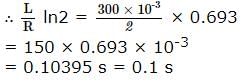AIIMS Full Mock Test 14 - NEET MCQ
30 Questions MCQ Test AIIMS Mock Tests & Previous Year Question Papers - AIIMS Full Mock Test 14
The plates of a capacitor are charged to a potential difference of 320 volts and are then connected across a resistor. The potential difference across the capacitor decays exponentially with time. After 1 second the potential difference between the plates of the capacitor is 240 volts, then after 2 and 3 seconds the potential difference between the plates will be
An electron remains undeflected when passing perpendicular to mutually perpendicular electric and magnetic fields. If the magnetic field is 8 Gauss and elecric field is 4000 V/m, the velocity of the electron is
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
An air capacitor of capacity C = 10 μ F is connected to a constant voltage battery of 12 volt. Now the space between the plates is filled with a liquid of dielectric constant 5. The charge that flows now from battery to the capacitor is
When light of wavelength 300 nm (nanometer) falls on a photoelectric emitter, photoelectrons are liberated. For another emitter, however light of 600 nm wavelength is sufficient for creating photoemission. What is the ratio of the work functions of the two emitters
A galvanometer of resistance 20 Ω is to be converted into an ammeter of range 1 A. If a current of 1 mA produces full scale deflection, the shunt required for the purpose is
In an inelastic collision between two bodies, the physical quantity that is conserved
In an L-R circuit, time constant is that time in which current grows from zero to the value
In an A.C. circuit, the potential difference across an inductance and resistance joined in series are respectively 16 V and 20 V. The total potential difference across the circuit is
Compare electrons accelerated through a certain potential difference and protons accelerated through the same p.d. If initial velocities are negligible, then the emergent
Gases begin to conduct electricity at low pressure because
When a proton is accelerated with 1 volt potential difference, then its kinetic energy is
A coil of inductances 300mH and resistance 2 Ω is connected to a source of voltage 2V. The current reaches half of its steady state value in
Dimensions of 1/μ₀ ∈₀, where symbols have their usual meanings, are
Minimum no. of 8 μF and 250 V capacitors, used to make a combination of 16 μF and 1000 V, is
A 2 μF condenser is charged to 500V and then its plates are joined through of consistance. The heat produced in the resistance in joules is
Two bodies of mass m₁ and m₂ have equal kinetic energies. If p₁ and p₂ are their respective momentum, then ratio p₁ : p₂ is equal to
There are four bodies having colours blue, red, black and white. When they are heated together and allowed to cool, which body will cool at the earliest?
A particle of mass m is moving in a horizontal circle of radius r under a centripetal force equal to - K/r2, where K is a constant. The total energy of the particle is
If two bulbs of wattage 25 and 30 W, each rated at 220 volts are connected in series with a 440 volt supply, which bulb will fuse?
A bar magnet of magnetic moment 3.0 A-m2 is placed in a uniform magnetic induction field of 2X 10–5 T. If each pole of the magnet experiences a force of 6 X 10–4 N, the length of the magnet is
When a magnetic needle is kept in a non-uniform magnetic field, it experiences :
A wire of length L and area of cross-section A is made of material of Young's modulus y. If the wire is stretched by the amount x, the work done is
A mass of 10 grams is suspended by a string and the entire thing is falling with a uniform acceleration of 400 cm/se2. The tension in the string will be (g=980 cm/sec2)
A particle of mass 10 gm is decribing simple harmonic motion along a straight line with period of 2 sec and amplitude of 10 cm. Its kinetic energy when it is at 5 cm from its equilibrium position is
In the following question, a Statement of Assertion (A) is given followed by a corresponding Reason (R) just below it. Read the Statements carefully and mark the correct answer-
Assertion(A): A charged particle moving in a uniform magnetic field penetrates a layer of lead and loses half of its kinetic energy. The radius of curvature of its path is now reduced to half of its initial value.
Reason(R): Kinetic energy is proportional to square of radius of curvature.
In the following question, a Statement of Assertion (A) is given followed by a corresponding Reason (R) just below it. Read the Statements carefully and mark the correct answer-
Assertion(A): A changing magnetic field acts as a source of electric field known as induced electric field.
Reason(R): Induced electric field can be produced by any static charge distribution.
In the following question, a Statement of Assertion (A) is given followed by a corresponding Reason (R) just below it. Read the Statements carefully and mark the correct answer-
Assertion(A): Frequency of note emitted by an organ pipe will decrease with rise in temperature.
Reason(R): Frequency = 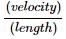
In the following question, a Statement of Assertion (A) is given followed by a corresponding Reason (R) just below it. Read the Statements carefully and mark the correct answer-
Assertion(A): The electron passing through crossed magnetic and electric field is always deflected from its path.
Reason(R): If velocity of electrons is equal to the ratio of electric and magnetic field applied then electron beam may remain undeflected.
|
3 videos|44 docs|66 tests
|
|
3 videos|44 docs|66 tests
|