AIIMS Full Mock Test 17 - NEET MCQ
30 Questions MCQ Test - AIIMS Full Mock Test 17
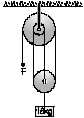
In the figure, at the free end of the light string, a force F is applied to keep the suspended mass of 18 kg at rest. Then the force exerted by the ceiling on the system (assume that the string segments are vertical and the pulleys are light and smooth) is: (g= 10 m/s2)
Aman stands on a weighingmachine kept inside a lift. Initially the lift is ascending with the acceleration ‘a’ due to which the reading is W. Now the lift decends with the same accleration and reading is 10 % of initial. Find the acceleration of lift ?
A monoatomic ideal gas, initially at temperature T1, is enclosed in a cylinder fitted with a frictionless piston. The gas is allowed to expand adiabatically to a temperature T2 by releasing the piston suddenly. If L1 and L2 are the length of gas column before and after expansion respectively, Then T1/T2 is given by:
The molecules of an ideal gas have 6 degrees of freedom. The temperature of the gas is T. The average translational kinetic energy of its molecules :
A mixture of ideal gasses N2 and He are taken in the mass ratio of 14 : 1 respectively. Molar heat capacity of the mixture at constant pressure is.
A non–conducting container is divided into two chambers that are separated by a valve. The left chamber contains one mole of a monatomic ideal gas. The right chamber is evacuated. At some instant, the valve is opened and the gas rushes freely into the right chamber.Which are of the following statements concerning this process is true?
For an ideal gas four processes are marked as 1, 2, 3 and 4 on P-V diagram as shown in figure. The amount of heat supplied to the gas in the process 1, 2, 3 and 4 are Q1 , Q2 , Q3 and Q4 respectively, then correct order of heat supplied to he gas is: [AB is process-1, AC is process-2, AD is adiabatic process-3 and AE is process-4]
Fig. shows graphs of pressure vs density for an ideal gas at two temperatures T1 and T2.
An ideal gas changes from state a to state b as shown n Fig. What is the work done by the gas in the process ?
If heat is supplied to an ideal gas in an isothermal process,
A given quantity of a gas is at pressure P and absolute temperature T. The isothermal bulk modulus of the gas is:
A liquidwith coefficient of volume expansion γ is filled in a container of amaterialhaving the coefficient of linear expansion α . If the liquid overflows on heating, then.
A metallic ball and highly stretched spring are made of the same material and have the same mass. They are heated so that they melt, the latent heat required
The relative humidity on a day,when partial pressure of water vapour is 0.012 × 105 Pa at 12°C is (take vapour pressure of water at this temperature as 0.016 × 105 Pa)
Power delivered to a body varies as P = 3 t2. Find out the change in kinetic energy of the body from t = 2 to t = 4 sec.
The transfer ratio B of a transistor is 50. the input resistance of the transistor when used inthe common emitter configuration is 1 k? The peak value of the collector AC current for an AC input voltage of 0.01 V peak is :
50 gm of copper is heated to increase its temperature by 100C same quantity of heat is given to 10 gm of water, the rise in its temperature is (Specific heat of copper = 42 joule-kg-1 0C-1)
A beaker contains 200 gm of water. the heat capacity of the beaker is equal to that of 20gm of water. The intial temperature of water in the beaker is 200C, if 440 gmof hot water at 920C is poured in it, the final temperature (neglecting radiation loss) will be nearest to :
On centrigrade scale the temperature of a body increases by 30 degrees. The increase in temperature on Fahrenheit scale is :
A vertical column 50 cm long at 50°C balances another column of same liquid 60 cm long at 100°C. The coefficient of absolute expansion of the liquid is :
A bar of iron is 10 cm at 20°C. At 19°C it will be ( α of iron =11 × 10-6/°C)
During the expansion process of a gas from 1m3 to 10 m3 while pressure changes accroding to equation P = 30V2 – 20V + 100. What is the work done by gas (in Joules) (Where pressure is in Pa and volume is in m3)
The ratio of specific heats of a gas is, then the numb r of degrees of freedom of the gas molecules for translational motion is:
Four containers are filled with monoatomic ideal gases For each container, the number of moles, the mass of an individual atom and the rms spee of the atoms are expressed in terms of n, m and vrms respectively. If TA, TB, TC and TD are their temperatures respectively then which one of the options correctly represents the order ?
In figure,P-V curve of an ideal gas is given. During the process, the cumulative work done by the gas
A fixed mass of gas undergoes the cycle of changes represented by PQRSP as shown in Figure. In some of the changes, work is done on the gas and in others, work is done by the gas. In which pair of the changes work is done on the gas?
A cylinder of capacity 20 litres is filled with H2 gas and the pressure of hydrogen in the cylinder is 5 × 106 N/m2. Then total average kinetic energy of translatory motion of its molecules is :
Two ideal gases at absolute temperature T1 and T2 are mixed. There is no loss of energy. The masses of the molecules are m1 and m2 and the number of moles in the gases are n1 and n2 respectively. The temperature of mixture will be.