AIIMS Full Mock Test 19 - NEET MCQ
30 Questions MCQ Test AIIMS Mock Tests & Previous Year Question Papers - AIIMS Full Mock Test 19
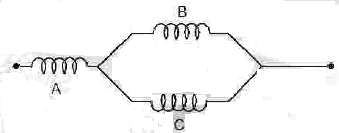
Three identical long solenoids A, B and C are connected as shown in the figure above. The magnetic field due to current flow is 2.0 T at the centre of solenoid A. What is the magnetic field at the centre of solenoid C ?
A metal wire loop is in a uniform magnetic field and the plane of the loop is perpendicular to the magnetic field. An e.m.f. will be induced in the loop if
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
If the rms current in a 50 Hz ac circuit is 5A, the value of the current 1/300 seconds after its value becomes zero is :
When a voltage measuring device is connected to AC mains, the meter shown the steady input voltage of 220V. This means
A rectangular coil of 300 turns has an average area of 25cm × 10cm. The coil rotates with a speed of 50 cps in a uniformmagnetic field of strength 4×10-2 T about an axis perpendicular of the field. The peak value of the induced e.m.f. is (in volt)
A solenoid has 2000 turns wound over a length of 0.30 meter. The area of its cross-section is 1.2×10-3 m2, Around its central section, a coil of 300 turns is wound. If an initial current of 2A in the solenoid is reversed in 0.25 sec, then the e.m.f. induced in the coil is
An ideal coil of 10 henry is joined in series with a resistance of 5 ohm and a battery of 5 volt. 2 sec after joining, the current flowing in ampere in the circuit will be
The adjoining figure shows two bulbs B1 and B2 resistor R and and inductor L.When the switch S is turned off
Two conducting circular loops of radii R1 and R2 are placed in the same plane with their centres coinciding. If R1 >> R2, the mutual inductance M between them will be directly proportional to
A thin semicircular conducting ring of radius R is falling with its plane vertical in a horizontalmagnetic induction B. At the position MNQ, the speed of the ring is V and the potential difference developed across the ring is
A uniform but time-varying magnetic field B(t) exists circular region of radius a and is directed into the plane of the paper, as shown. The magnitude of the induced electric field at point P at a distance r from the center of the circle region
When a certain circuit consisting of a constant e.m.f. E an inductance L and a resistance R is closed, the current in, it increases with time according to curve 1. After one parameter (E,L or R) is changed, the current in the circuit increases with time according to curve 2. Which parameter was changed and in what direction
When the number of turns and the length of the solenoid are doubled keeping the area of cross section same, the inductance
In the ideal oscillating circuit, the capacitance of the capacitor is 25 μF and has initial charge of 30 μC. The inductance of coil is 0.04 H. The maximum magni de of current in the circuit, after closing the switch K is
An alternating current of frequency ‘f’ is flowing in a circuit containing a resistance R and a choke L in series. The impedance of this circuit is
A resonant ac circuit contains a capacitor of capacitance 10-6 F and an inductor of 10-4 H. The frequency of electrical oscillations will be
A resistance of 300 Ω and an inductance of 1/π henry are connected in series to a ac voltage of 20 volts and 200 Hz frequency. The phase angle between the voltage and current is
An alternating voltage E = 200√2 sin (100 t) is connected to a 1 microfarad capacitor through an ac ammeter. The reading of the ammeter shall be
A 220 V, 50 Hz ac source is connected to an inductance of 0.2 H and a resistance of 20 ohm in series. What is the current in the circuit
In the circuit shown below, the ac source has voltage V = 20 cos (ωt) volts with ω = 2000 rad/sec. The amplitude of the current will be earest to
In a circuit L,C and R are connected in series with an alternating voltage source of frequency f . The current leads the voltage by 45°. The value of C is
Which one of the following curves represents the variation of impedance (Z) with frequency f in series LCR circuit
A constant voltage at different frequencies is applied across a capacitance C as shown in the figure. Which of the following graphs
Correctly depicts the variation of current with frequency?
The figure shows variation of R, XL and XC with frequency f in a series L, C, R circuit. Then for what frequency point, the circuit is inductive
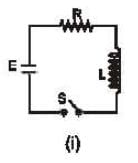
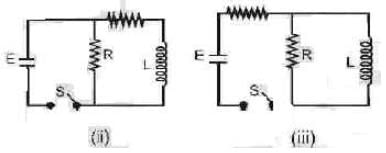
In which of the following circuit is the current maximum just after the switch S is closed
A small coil is introduced between the poles of an electromagnet so that its axis coincides with the magnetic field direction. The number of turns is n and the cross sectional area of the coil is A.When the coil turns through 1800 about its diameter, the charge flowing through the coil is Q. The total resistance of the circuit is R. What is the magnitude of the magnetic induction
Two circular coils A and B are facing each other as shown in figure. The current i through A can be altered
A conducting loop having a capacitor is moving outward from the magnetic field then which plate of the capacitor will be positive
A straight wire of length L is bent into a semicircle. It is moved in a uniform magnetic field with speed v with diameter perpendicular to the field. The induced emf between the ends of the wire is
If in a coil rate of change of area is and current become 1 amp form 2 amp in 2 x 10-3sec. If magnetic field is 1 Tesla then self inductance of the coil is
|
3 videos|44 docs|66 tests
|
|
3 videos|44 docs|66 tests
|

















