AIIMS Full Mock Test 20 - NEET MCQ
30 Questions MCQ Test AIIMS Mock Tests & Previous Year Question Papers - AIIMS Full Mock Test 20
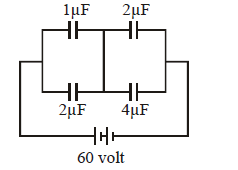
The charge stored in the 4 μF capacitor in the electric circuit shown is
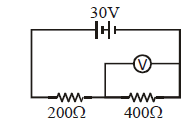
The voltmeter shown in the circuit has resistance of 400 Ω. Its reading will be
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
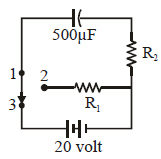
A capacitor of capacity 50 μF is connected to a constant source of emf 20 volt as shown in figure. Here R2 = 2 KΩ. The amount of heat produced in R1 = 3 KΩ, when the key is shifted from contact 3 to 2, is
A charged particle is moving in a uniform magnetic field in a circular path of radius R. When energy of the particle is doubled, then new radius will be
In a series LCR circuit the frequency of a 20 V AC voltage source is adjacent in such a fashion that the reactance of the inductor measures 30Ω and that of the capacitor is 22 Ω. If resistance R is 6 Ω, then the potential difference across the series combination of L and C will be
A perfectly reflecting mirror has an area of 2 cm2. Light energy is allowed to fall on it for 30 minute at the rate of 15 × 104 watt/m2. The force that acts on the mirror is
A small object is placed at a distance of 8 cmfrom a convex mirror of radius of curvature 24cm. The magnification of the image obtained is
A ray of light travels from an optically denserto a rarer medium. The critical angle for thetwo media is C. Without total internal reflectionthe maximum possible deviation of the raywill be
A plano-convex lens has refractive index 1.4and its radius of curvature is 40 cm. The focal length of the lens is
A telescope has an objective lens of focal length1 m and an eye piece with focal length 2 cm. Ifthis telescope is used to see a 25 m tall building at a distance of 1 km, then the height of theimage of the building formed by the objectivelens would be
In the following diagram all the wires are of same material and have uniform cross-section. The magnitude of magnetic field at the centre O of the square due to arm AB is B1 and dueto the arm CD is B2 then the ratio B1/B2 IS
After the half lives, 8 g of radioactive material remains in a sample. What will be the amount of substance at t = 0 s ?
In the given figure a convex lens and concave mirror are separated by 40 cm. Position of final image is (from the pole of mirror)
In YDSE the fringe width is 0.06 mm. If thewavelength of light used is increased by 25%and the slit separation is decreased by 25% then fringe width will be
An unpolarised light of intensity I0 is incidenton a pair of two polaroids held coaxially such that their transmission axes make an angle of 30° with each other. The fraction of intensity oflight emerging from the pair is
The refracting angle of a prism is A and thecritical angle of the medium of the prism is QC.There will be no emergent ray when
If λ is de-Broglie wavelength of electron movingin nth orbit, then the radius of 3rd orbit in hydrogen atom is
In a common emitter configuration collectorcurrent and emitter current are 3.09 mA and 3.19 mA respectively. The current gain in this configuration is
An arc of radius R is charged. The linear density of charge is λ and the arc subtends an angle 3/π at the centre. What is electric potential at the centre ?
In photoelectric effect, the electrons are ejectedfrom metals if the incident light has certain maximum
Stopping potential in photoelectric experimentfor a given metal surface
A man runs towards a stationary plane mirror with velocity 15 m/s. Magnitude of velocity of the man relative to his image is
If ratio of amplitudes of two coherent sources producing an interference pattern is 3 : 4, then ratio of intensities at maxima and minima is
The sun maintains its brightness due to which of the following reactions in its core ?
The equivalent resistance between P and Q in the network shown is
Torque on a current carrying coil is maximumin a uniform magnetic field, when
|
3 videos|44 docs|66 tests
|
|
3 videos|44 docs|66 tests
|

















