AIIMS Full Mock Test 21 - NEET MCQ
30 Questions MCQ Test AIIMS Mock Tests & Previous Year Question Papers - AIIMS Full Mock Test 21
A body moves along a straight line such that its displacement at any time t is given by s=(t3 - 6t2 +3t+4) The velocity of body when acceleration is zero is
The north pole of a magnet is brought near a metallic ring. Then the direction of the induced current in the ring will be
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
When a disc having velocity of its center of mass vcm, is rolling then velocity at the highest point on the disc will be
If 92U238 undergoes successively 8α - decays and 6β - decays then the resulting nucleus is
The equation of wave is given by
If the displacement is 5 cm at t = 0 then the total phase at t = 7.5 sec is
A wooden block of mass 8 kg is tied to a string attched to the bottom of the tank. In the equilibrium the block is completely immersed in water. If relative density of wood is 0.8 and g = 10 ms–2, the tension T, in the string is
In a thermodynamic process, pressure of a fixed mass of gas is changed in such a manner that the gas molecule will give out 30 joule of heat and 10 joule of work is done on the gas. If the initial internal energy of the gas was 40 joule then the final internal energy will be
A hollow charged metal sphere has radius r. If the potential difference between its surface and a point at a distance 3r from the center is V, then electric field intensity at a distance 3r is
A source of sound is moving with a velocity 50 m/s towards a stationary observer. The observer measures the frequency of the source as 1000 Hz. What will be apparent frequency of the source when it is moving away from the observer after crossing him? The velocity of sound in the medium is 350 m/s.
The focal length of a thin convex lens for red and blue rays are 100 cm and 96.8 cm respectively. The dispersive power of the material of the lens is
Two particles are seen to collide and move jointly together after the inelastic collision. During such a collision, for the total system
A capillary tube of internal diameter 2 mm and external diameter 5 mm hangs vertically from the arm of the balance. The lower end of the tube just touching a liquid of surface tension 40 dyne/cm. If the angle of contact is zero, the change in weight due to surface tension will be
The pressure inside the soap bubbles is 1.01 and 1.02 atmosphere respectively. The ratio of their volumes is
The angular velocity of rotation of a star (of mass M and radius R) at which the matter starts to escape from its equator is
For an enclosure maintained at 1000 K the minimum radiation occurs at wavelength . If the temperature is raised to 2000 K, the wavelength will be
A wave traveling along the negative x-direction with amplitude 0.01 m. frequency 550 Hz and speed 330 m/sec is represented by the equation
The given figure representing a sinusoidal wave is traveling in string along positive x-direction. P and Q are the two points on the string. The direction of the velocity of particles at P(VP) and at Q(VQ) will be
Calculate the time period of the block of mass m shown in figure, if the spring constant of the spring is K and surface is frictionless
A projectile is given initial velocity 50 ms–1 and angle of projection is 30º. The average acceleration in first three seconds will be
What is maximum value of ‘F’ for which both blocks move together? (g = 10 m/sec2)
If coefficient of limiting friction is 2.4 then maximum uniform speed of a vehicle on a track of radius 24 m for no slipping, can be (Take g = 10 m/sec2)
The minimum ‘v’ that should be given to block so that it doesn’t slide on smooth circular track i.e., it breaks off immediately is
Escape velocity; 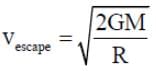
here r→ radius of earth
M → mass of earth
and G → universal constant
% error in measurement of vesc is 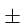 3% and in 'M' and 'R' are
3% and in 'M' and 'R' are 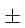 1% and
1% and 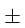 2% respectively. The maximum % error in measurement of 'G' is
2% respectively. The maximum % error in measurement of 'G' is
A missile is launched with the velocity less than the escape velocity. Sum of its kinetic energy and potential energy is
The bob of pendulum is attached to a metallic thread having coefficient of linear expansion ‘α’. If temperature is increased by 50º C and α = 1.2 x 10–3 (Cº)–1, then in 5 minutes the pendulum.
One mole of oxygen gas undergoes 50% dissociation into oxygen atoms. The degree of freedom of mixture will be
A particle of charge +q and mass m moving under the influence of a uniform electric field and a uniform magnetic field
follows trajectory from P to Q as shown in figure. The velocities at P and Q are
and
respectively. Which of the statement(s) is/are correct
Two straight long conductors AOB and COD are perpendicular to each other and carry currents l1 and l2. The magnitude of the magnetic induction at a point P at a distance a from the point O in a direction perpendicular to the plane ABCD is
|
3 videos|44 docs|66 tests
|
|
3 videos|44 docs|66 tests
|

















