AIIMS Full Mock Test 22 - NEET MCQ
30 Questions MCQ Test AIIMS Mock Tests & Previous Year Question Papers - AIIMS Full Mock Test 22
A solid body initially at 0°C is being heated by a constant power source and variation of temperature (T in °C) with time t (in S) in shown in figure. If S1 is specific heat of body in solid phase & S2 is specific heat in liquid phase, then which is correct

During adiabatic free expansion of a an ideal gas, its temperature
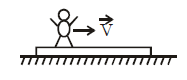
A plank of mass M is lying on a horizontal frictionless surface. A man of mass m moves on plank with velocity  relative to plank in horizontal direction. Then velocity of plank relative to surface is
relative to plank in horizontal direction. Then velocity of plank relative to surface is
Which of the following pattern of electrostatic field line is possible ?
A particle moving in the xy plane according to the equations ( in S.I. units), x = 4t2 + 5t + 16, y = 5t. The acceleration of the particle is
Two satellites orbit the earth, as shown. Their orbits are circular, and each satellite travels at a constant speed. If the mass of satellite #2 is twice the mass of satellite #1, which satellite’s speed is greater?
In which of the following, the emitter base junction is forward biased?
The diode used in the circuit shown in the figure has a constant voltage drop of 0.5 V at all currents and a maximum power rating of 100 milliwatts. What should be the value of the resistor R, connected in series with diode for obtaining maximum current ?
Photoelectrons are being obtained by radiation of wavelength 3100 Å. In order to increase the kinetic energy of ejected photoelectrons
If W1, W2 and W3 are represent the work done in moving a particle from A to B along three different paths 1, 2 and 3 respectively (as shown) in the gravitational field of a point mass m, find the correct relation between W1, W2 and W3
A thin circular ring of mass `M and radius r is rotating about its axis with a constant angular velocity w, Two objects, each of mass m, are atached gently to the opposite ends a diameter of the ring. The wheel now rotates with an angular velocity
One quarter sector is cut from a uniform circular disc of radius . This sector has mass M. It is made to rotate about a line perpendicular to its plane and passing through the centre of the original disc. Its moment of inertia about the axis of rotation is
For an object travelling in a straight line, its velocity (v, in m/s) as a function of time (t, in s) is given by the following graph
Which graph best illustrates the object’s distance from its starting point?
An astronaut standing on the surface of the moon (mass = M, radius R) holds a feather (mass = m) in one hand a hammer (mass = 100m) in the other hand, both at the same height above the surface. If he release them simultaneously, what is the acceleration of the hammer?
The diagram refers to the collision of two blocks on a frictionless table. Before the collision, the block of mass m is at rest
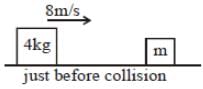
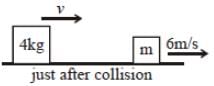
If the collision were elasticelastic, what is the total kineti energy of the block just after the collision ?
The figure shows two positively charged particles. The +Q charge is fixed in position, and +q charge is brought close to +Q and released from rest. Which of the following graphs best depicts the acceleration (a) of the +q charge as a function of its distance (r) from +Q?
Which of the following statements is true concerning phase changes?
Refer to the circuit shown above. All six resistors in the circuit have the same resistance R, and the battery provides a source of constant voltage V. Which one the following changes to the circuit would decrease the power dissipated by resistor?
An object of mass 5 kg is acted upon by exactly four forces, each of magnitude 10N. Which of the following could not be the resulting acceleration ofthe object?
A rope stretched between two fixed points can support transverse standing waves. What is the ratio of the sixth harmonic frequency to the third harmonic frequency?
In which of the following situations, involving source of sound and a detector of the sound, it is possible that there is no perceived Doppler shift?
If a 50 kg block of solid marble (specific heat = 0.9 kJ/kg°C), originally at 20°C, absorbs 100 kJ of heat, which one of the following best approximates the temperature increase of the marble block?
A sample of an ideal gas is heated, doubling its absolute temperature. Which of the following statements best describes the result of heating the gas?
Which of the following changes to a double-slit interference experiment with light would increase the widths of the fringes in the interference pattern that appears on the screen?
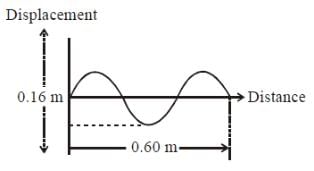
The drawing shows the displacement of a traveling wave at time t = 0. If the wave speed is 0.5 m/s and the wavelength is λ meter, what is the period of the wave (in seconds)?
During each cycle, a heat engine takes in 600 J of energy from its high temperature source and ejects 400 J of exhaust heat into its low temperature sink.
What is the efficiency of this heat engine?
Three point charges are arranged along a straight line as shown here. If k denotes coulomb’s constant, what is the strength of the electrostatic force felt by the positive charge at the left end of the line?
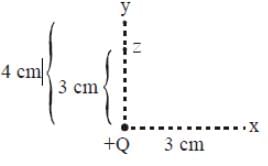
How much work is done by the electric field of the stationary charge +Q = +2C to move a charge of 1 × 10–9C from position X to position Z?
The value of coulomb's constant, k is 9 x 109 N.m2/C2
|
3 videos|44 docs|66 tests
|
|
3 videos|44 docs|66 tests
|


















