AIIMS Full Mock Test 23 - NEET MCQ
30 Questions MCQ Test AIIMS Mock Tests & Previous Year Question Papers - AIIMS Full Mock Test 23
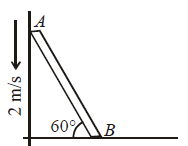
In the figure shown a rod of length L is inclined at an angle 60° with the floor against a smooth wall. If the end A moves with a velocity 2 m/s, the end B should move with a velocity
When charged particle rotates in a circular path the ratio of its magnetic moment to its angular momentum is equal to
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
A capacitor 100μF, and inductor 16H and a resistor 100 Ω are connected in series across an ac source of E = 200 sin 25t the power dissipated in the circuit is
Two charged particles Y and Z enter a space of uniform magnetic field with equal velocities perpendicular to the magnetic field. The path followed is shown below. Then
The graph showing the variation of terminal velocity v of spherical balls with the square of radius (r) in a viscous liquid can be
Three vectors 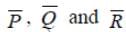 arranged in a region such that
arranged in a region such that  The vectors must be
The vectors must be
A particle executes SHM. Its velocities are V1 and V2 at displacement x1 and x2 from mean position respectively. The frequency of oscillation
A body of density ρ is floating at the interface of two immisible liquids of densities ρ1 and ρ2 respectively. Fraction of volume inside upper liquid is
A radio active material decays by simultaneous emission of two particles with respective half lives 1620 and 810 years. The time (in years) after which one fourth of the material remains
The radii of two planets are respectively R1 and R2 and their densities are P1 and P2. Ratio of acceleration due to gravity at their surface will be
Figure shows a force distance graph of a particle moving along a straight line due North. The KE of the particle at a distance of 3m from the starting point is
One end of a spring of force constant K is fixed to a vertical wall and the other to a body of mass m resting on a smooth horizontal surface. There is another wall at a distance x0 from the body. The spring is then compressed by 2x0 and released. The time taken to strike the wall is
The area swept by a planet revolving around the sun in time t is given as A = A0 + Kt, where A0 and K are the constants. If the mass of the planet is m, then angular momentum of the planet about the sun will be
The height of image formed by a convex lens on a screen is 8 cm. For the same position of object and screen a sharp image of height 12.5 cm is formed. The height of the objects
A spring has an unstreched length of 8 cm and when a weight is hung on it, its length becomes 14 cm. The time period of oscillation of the weight is
Three consecutive frequencies of standing wave in an organ pipes are 165 Hz, 275 Hz and 385 Hz. If velocity of sound in organ pipe is 330 m/s, then length of organ pipe is
If represents electric flux then dimension of the quantity
is same as that of
If M is mass of a thin semicircular ring of radius R. Moment of Inertia of this thin semicircular ring about axis passing through its C.M. and perpendicular to its plane is
If a particle of mass m is at a height h =R/4 where R is radius of the earth. Potential energy of particle with respect to reference level on surface of the earth is
A block of mass m is initially at rest on a rough horizontal surface. A horizontal force varying with time F = αt2 is applied on the block as shown in figure. Which of the following graph represents variation of magnitude of frictional force Ff on block with time t
A particle moves on X-axis of a reference frame with velocity varying with x-co-ordinate of particle as shown in graph. Acceleratin of particle at x = 5m is
For the circuit shown in figure no current flows through cell of emf ∈ . Then R is equal to
A nucleus splits into two nuclear parts having radii in the ratio 1 : 2. Their velocities are in the ratio
In the diagram, the input is across the terminals A and C and the output is across B and D. Then output is
Power of a lens in air is +2D. If refractive of material of lens is 3/2 and the lens is dipped in water (refractive index 4/3), then its power becomes
Angle of a prism is 30° and its one surface is silvered. Light ray falling at an angle of incidence 60° on the first surface return back through the same path after suffering reflection at the silvered surface. Refractive index of material of prism is
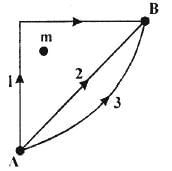
29. If W1, W2 and W3 are represent the work done in moving a particle from A to B along three different paths 1, 2 and 3 respectively (as shown) in the gravitational field of a point mass m, find the correct relation between W1, W2 and W3
One quarter sector is cut from a uniform circular disc of radius . This sector has mass M. It is made to rotate about a line perpendicular to its plane and passing through the centre of the original disc. Its moment of inertia about the axis of rotation is
|
3 videos|44 docs|66 tests
|
|
3 videos|44 docs|66 tests
|

















