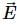AIIMS Full Mock Test - 9 - NEET MCQ
30 Questions MCQ Test AIIMS Mock Tests & Previous Year Question Papers - AIIMS Full Mock Test - 9
A coil of inductance 40 H is connected in series with a resistance of 8 Ω and the combination is joined to the terminals of a 2 volt battery. The time constant of the circuit is
In photoelectric phenomenon, the number of photo electrons emitted depends on
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
An air capacitor of capacity C = 10μ F is connected to a constant voltage battery of 12 volt. Now the space between the plates is filled with a liquid of dielectric constant 5. The charge that flows now from battery to the capacitor is
Identify that set in which all the three sustances are good conductors of electricity
Photons of energy 6eV are incident on a metal surface whose work function is 4eV. The minimum kinetic energy of the emitted photoelectrons will be
A shell is fired from a canon with velocity v m/sec at an angle θ with the horizontal direction. At the highest point in its path it explodes into two pieces of equal mass. One of the pieces retraces its path to the cannon and the speed in m/sec of the other piece immediately after the explosion is
What is the de Broglie wavelength of the α-particle accelerated through a potential difference V
A metal plate gets heated, when cathode rays against it, due to
The power factor of a series LCR circuit when at resonance is
The magnetic flux of 500 micro-webers passing through a 200 turn coil is reversed in 20 x 10-3 seconds. The average e.m.f. induced in the coil in volts is
In an oscillating LC circuit the maximum charge on the capacitor is Q. The charge on the capacitor when the energy is stored equally between the electric and magnetic fields is
A long spring is stretched by 2 cm, its potential energy is U. If the spring is stretched by 10 cm, the potential energy stored in it will be
A particle of mass m is moving in a horizontal circle of radius r under a centripetal force equal to - K/r2, where K is a constant. The total energy of the particle is
The initial temperature of a body is 80ºC. If its temperature falls to 64ºC in 5 minutes and in 10 minutes to 52ºC then the temperature of surrounding will be
An infinite long plate has surface charge density σ . As shown in the figure a point charge q is moved from A to B. Net work done by electric field is:
An electric lamp is marked 60 W, 220 V. The cost of kilo watt hour of electricity is Rs 1.25. The cost of using this lamp on 220 V for 8 hours is
An electric dipole has the magnitude of its charge as q, and its dipole moment is p. It is placed in a uniform electric field E. If its dipole moment is along the direction of the field, then force acting on it and its potential energy are respectively
For protecting sensitive equipment from external magnetic field, it should be
A bar magnet of pole strength 20 A-m is divided into 4 equal parts by slicing it along lines parallel to its length and breadth, passing through centre. The pole strength of each piece is
Two wires of the same material and length, but diameters in the ratio 1 : 2 are stretched by the same force. The elastic potential energy stored per unit volume for the two wires when stretched, will be in the ratio of
When a train stops suddenly, passengers in the running train feel an instant jerk in the forward direction because
To impart high energy to sub atomic particles, the particle accelerator used is
A body of mass 2 kg is made to oscillate with an angular frequency 4 rad-s⁻1 on a spring. The force constant of the spring is
In the following question, a Statement of Assertion (A) is given followed by a corresponding Reason (R) just below it. Read the Statements carefully and mark the correct answer-
Assertion(A): A particle of mass m collides with another stationary particle of mass M. If the particle m stops just after the collision, the coefficient of restitution of collisions is equal to 1.
Reason(R): Momentum of system just before and after the collision remains constant.
In the following question, a Statement of Assertion (A) is given followed by a corresponding Reason (R) just below it. Read the Statements carefully and mark the correct answer-
Assertion(A): Air quickly leaking out of a balloon becomes cooler.
Reason(R): The leaking air undergoes adiabatic expansion. Reason(R): The leaking air undergoes adiabatic expansion.
In the following question, a Statement of Assertion (A) is given followed by a corresponding Reason (R) just below it. Read the Statements carefully and mark the correct answer-
Assertion(A): Coulomb's law gives the electric field due to a distribution of charges , while Biot-Savarts law gives the magnetic field due to a current element .
Reason(R): A stationary charge generates an electric field while a current generates a magnetid field in the surrounding space.
In the following question, a Statement of Assertion (A) is given followed by a corresponding Reason (R) just below it. Read the Statements carefully and mark the correct answer-
Assertion(A): A lens of water formed by very thin convex glass layer has lesser focal length than a double convex lens of glass.
Reason(R): Refractive index of glass is more than that of water.
|
3 videos|44 docs|66 tests
|
|
3 videos|44 docs|66 tests
|


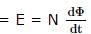
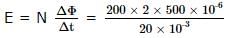 = 10 volts
= 10 volts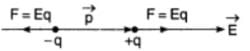
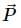 is parallel to
is parallel to